Characteristic Reactions of Aluminum Ions (Al³⁺)
- Page ID
- 97259
- Most common oxidation state: +3
- M.P. 648º
- B.P. 1800º
- Density 2.70 g/cm3
- Characteristics: Silvery, rather soft. Very active, but protected by an oxide coating.
- Characteristic reactions of \(\ce{Al^{3+}}\):
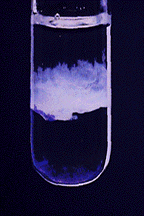
Aqueous Ammonia:
Aluminum ion reacts with aqueous ammonia to produce a white gelatinous precipitate of Al(OH)3:
\[\ce{Al^{3+}(aq) + 3NH3(aq) + 3H2O(aq) <=> Al(OH)3(s) + 3NH4+(aq)} \nonumber \]
Sodium Hydroxide
A strong base, such as \(\ce{NaOH}\), precipitates \(\ce{Al(OH)3}\), which is amphoteric and dissolves in an excess of hydroxide or in acids.
\[\ce{Al^{3+}(aq) + 3OH^-(aq) <=> Al(OH)3(s)} \nonumber \]

\[\ce{Al(OH)3(s) + OH-(aq) <=> Al(OH)4-(aq)} \nonumber \]
\[\ce{Al(OH)3(s) + 3H+(aq) <=> Al3+(aq) + 3H2O(l)} \nonumber \]

Aluminon
The dye aluminon is adsorbed by the gelatinous \(\ce{Al(OH)3}\) precipitate to form a red "lake" and a colorless solution. Although this reaction is not suitable for separation of aluminum ion, it can be used as a confirmatory test for \(\ce{Al^{3+}}\) after precipitation of \(\ce{Al(OH)3}\) with aqueous ammonia.

No Reaction
\(\ce{Cl^-}\), \(\ce{SO_4^{2-}}\)


