Gases for GC, parameters
- Page ID
- 61151
Gases used in gas chromatographic analyses are divided into
- Carrier gases The carrier gases are responsible for the transport of the sample components through the system and the detector gases for the detector performance.
- Detector gases. In certain situations the same gas can be applied as carrier gas and detector gas.
The most commonly used gases are nitrogen, helium, hydrogen and argon/methane.
A number of factors play a role in the choice of carrier gas:
- Gas supply
- Viscosity
- Purity
- Reactivity
- Detection method
- Safety
Carrier Gas Determines Detector
The type of carrier gas used for the separation often determines which detector is appropriate.
The most widely used GC detector is the flame ionization detector (FID), which is compatible with carrier gases like nitrogen, helium as well as hydrogen. Hydrogen has the advantage that it can be employed as carrier gas as well as detector make-up gas. In addition to detector gas, the FID also needs oxygen and air for combustion.
Comparison of GC Detectors
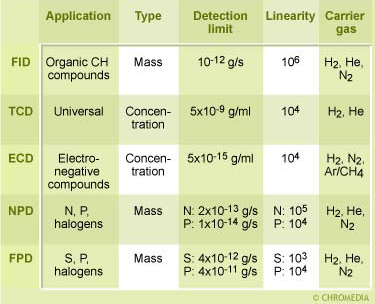
The most universal detector, the TCD or katharometer, works best with hydrogen or helium, but this choice depends on the type of analytes. Thermal conductivity of hydrogen and helium are much larger compared to nitrogen and organic components, as a result of which the detector is more sensitive.
The electron capture detector (ECD) requires an argon-methane mixture.
It is quite obvious which carrier gas should be used with an argon ionization detector or a helium detector. The operation of the various detectors and the problems likely to occur will be discussed elsewhere.
Viscosity
Gases like liquids, exhibit a certain viscosity ('flow thickness'). This is determined by the density of the gas, which in turn is dependent on pressure and temperature as well as the molecular structure (molecular weight). Hydrogen, for example, has a lower viscosity at a certain temperature and pressure than nitrogen, helium and argon. The viscosity determines the required pressure to obtain a certain gas flow through the column. The viscosity can, therefore, become an important feature when long columns are used.
Temperature Effect on Gas Viscosity
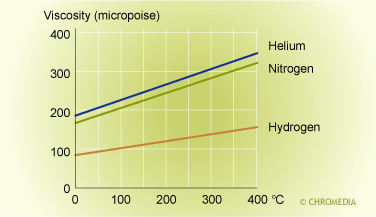
In particular, for packed analytical and preparative columns longer than 5 meters and capillary columns of 50 meters and longer, it is recommended to choose carrier gases with a low viscosity, for instance hydrogen in order to avoid damaging parts of the system (pressure gauges, connections, separation column).
Purity
Carrier gases should exhibit a high enough purity. Reactive components, including water and oxygen, can seriously damage the stationary phase.
- Oxygen can lead to oxidation resulting in a change in retention, loss of resolution and peak tailing.
- Water can damage the stationary phase, the sample components and the support material as a result of hydrolysis. This becomes evident in baseline noise and increased peak tailing.
Purity and Price of Carrier Gases (Indicative)

Not only carrier gases but also detector gases must be sufficiently pure. Especially compressed air, which can contain trace organic compounds can be a source of noise in the flame ionization detector.
Presently most carrier gases can be supplied commercially with a purity of 99.999% (purity code 5.0).
Reactivity
Carrier gases should be sufficiently inert. They must neither cause damage to the stationary phase nor to the sample components.
Chemical change can lead to a substantial difference in retention times. One should also bear in mind that the external conditions for a chemical reaction to occur are affected by high temperatures in the column as well as in the injector.
Oxidation of Stationary Phase (Reactivity)
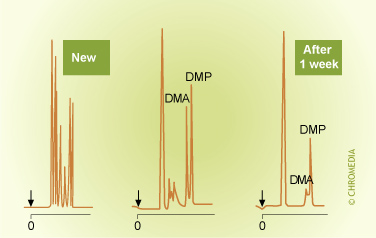
Well-purified carrier gases such as helium and nitrogen will generally present no problems. Caution should be used with hydrogen. Although hydrogen offers many advantages as a carrier gas, it is less inert than the other gases. In particular strongly unsaturated organic compounds are sensitive to addition reactions with hydrogen.
Safety
With regard the safety of a GC system and carrier gas we have to address the fact that hydrogen is extremely inflammable and mixtures of, for instance 4%, of hydrogen with air (oxygen), are explosive.
Safety is ensured as long as hydrogen can be operated in a closed gas system. However, a GC gas system can be never completely sealed. At the outlet, the gas is instantaneously burned in the FID.
In case of leakages elsewhere in the system, the situation can become unsafe. Fortunately, hydrogen leaks can be quickly and effectively detected by alarm systems, notably in the small compartment of the GC-oven.
Hydrogen safety system:
- Continually checks air inside GC oven for presence of hydrogen
- Alarm sounds when hydrogen is above a defined concentration
- Power & gas flow is shut-off
- LCD readout of hydrogen concentration
- Better than 10% accuracy

