Qualitative analysis: identification
- Page ID
- 61091
Nico Vonk, Avans+, Breda, The Netherlands
Abstract
A qualitative analysis involves the identification of a component by means of peak data on the chromatogram. The retention of a component is the result of a specific interaction of that component with the stationary phase and the mobile phase. Since the retention time is a specific property of a component, it may be used as a means to identify the component.
Level: Basic
Three factors should be considered:
- The retention time is no fixed property of a component, but it is the result of the behavior of a component in a system, in which the system contributes substantially to the retention time.
- The retention time does not offer any conclusive information concerning identification. Without any prior knowledge about the component, no identification can be made. At best an assumption can be expressed about the identity of the component. This is valid for a chromatographic analysis in general. A 100% identification is not possible in chromatography. It will always be an assumption which depends on prior knowledge of the component, the efficiency of the system and the identification method used.
- Unambiguous identification of a compound in GC is only possible using a spectroscopic identification method, the most commonly being mass spectrometry. Such additional techniques must be applied to identify the peaks or to supply information about the nature and the molecular structure of the compounds.
We can identify a compound:
- By retention time
- total retention time
- relative retention
- retention indices
- By spectroscopic techniques (MS, IR, NMR). Especially spectroscopic techniques are very useful, such as (on-line) mass spectroscopy (GC-MS), infrared (IR) and nuclear magnetic resonance (NMR). GC-MS can provide very selective and sensitive detection and positive (legal) identification of the compounds of interest. This is important in pharmaceutical research, forensic applications and doping control.
By Retention Time
The retention time of the unknown component is compared to the retention time of a so-called standard. This is a compound of which the identity is known and which is likely to possess the same identity as the unknown component. When the retention times of both compounds are similar, the unknown is considered identified. If the analyte itself is not available as a pure substance, identification based on chromatographic results only is not possible.
Identification by Retention Time

The analyst should always realize that the retention time is not only dependent on the component but also on the system (column, stationary phase, conditions and the instrumental settings and performance). It means that a correct comparison is only possible when two chromatographic runs are performed under identical conditions on the same GC system. Only under these circumstances the differences in retention times due to the stationary phase, the mobile phase, the flow, the column length, the column temperature etc. can be ignored. Validation of the system can prove the performance and stability of the instrument, using statistics to show its robustness and reproducibility.
By Relative Retention Times
Identification by Relative Retentions
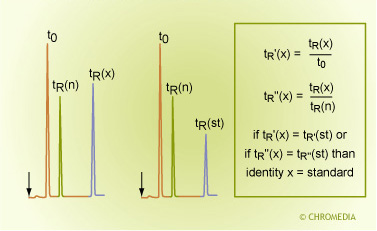
This method is very similar to the identification by retention times. Sometimes results must be compared between runs on different systems, times or even laboratories. The retention time of the unknown component is also compared to a reference compound in the mixtures (here denoted as ‘n’). In order to eliminate carrier gas flow fluctuations or differences in column lengths, not the absolute retention times are compared but the relative retention times.
The retention times is related to the t0 peak or the retention time of another component - an internal standard - which has been added to the sample.
By Retention Index
In the retention index method the retention time of an unknown compound is compared to a well chosen reference compound or group of compounds. The retention of the unknown is expressed in terms of the retention of this single compound or group of compounds. The unknown can be identified by comparing its index to similar indices of compounds listed in literature.
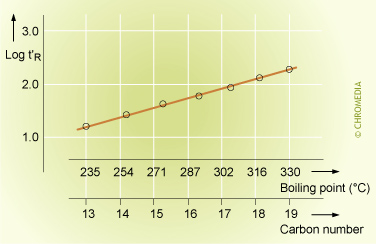
Homologous series
The linear relationship between the log value of the retention time and the carbon number of the components of a homologous series during an isothermal analysis is well known in chromatography. It reflects the logarithmic relationship between the boiling points of the components of a homologous series and their carbon number. We can use this relationship to identify members of a homologous series without injecting a reference sample of the actual compound. This is very useful in the analysis of n-alkanes, n-alcohols, FAME’s, etc.
Relation Between Retention, Boiling Point and Carbon Number
The most well known retention index system is the one developed by Kovats.
By Kovats index
The most well known retention index system is the one developed by Kovats. He developed an identification system whereby an index has been linked to the retention time. The index relates to a fixed group of reference compounds. If the Kovats index is determined for an unknown sample and is then compared to published indices of known components, identification can be made.
Kovats introduced an index system in which the retention of the unknown was related to the homologous series of the normal alkanes. It makes explicit use of the linear relationship between the log value of the retention time and the carbon number of the components of a homologous series.
Kovats Index
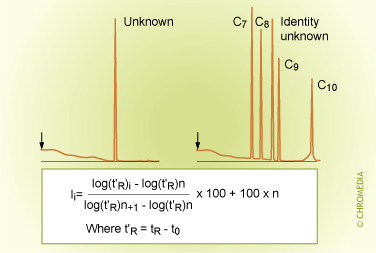
He defined the index of a normal alkane as its carbon number multiplied by the factor 100. This means that propane, for instance, has the index I = 300 whatever the conditions. Pentane has index: 500, octane: 800, decane: 1000 and dodecane: 1200 etc. All other components are related to this. If, for example, a certain compound under certain chromatographic conditions would have a retention time between that of pentane and hexane, it has a Kovats index between 500 and 600. A compound eluting between undecane and dodecane has an index between 1100 and 1200 etc.
This unique index is dependent primarily on the type of stationary phase. Except for polar components on a polar phase the index is also practically independent on temperature but is independent of film thickness, column length, column diameter and carrier gas velocity. These parameters are completely ruled out in the Kovats index.
Identification by Kovats index
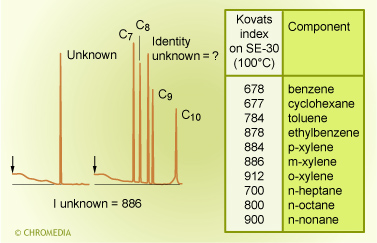
A general procedure for the calculation of the Kovats index is as follows: On the basis of the retention time of an unknown component and the retention times of the normal alkanes, which have been recorded under the same chromatographic conditions, the Kovats index is calculated with the aid of the formula above. Note that Z and Z+1 are the carbon numbers of the alkanes, which elute just before and just after the sample component (x). t' is the net retention time (thus tR - t0). After calculating the index, it can be compared to the values in literature in order to identify the unknown compound. It is not really necessary to know the identity of a component in order to use a retention index method. Some knowledge about the identity can improve the reliability of the identification considerably.
The reason that the Kovats index in the formula uses the logarithms of the retention times is because there is a linear relationship between the logarithms of the retention times and the carbon number of the components from a homologuous series. This is the result of the logarithmic relationship between the boiling points of the components of a homologuous series and their carbon number.
Identification of a Mixture
Fingerprint Analysis
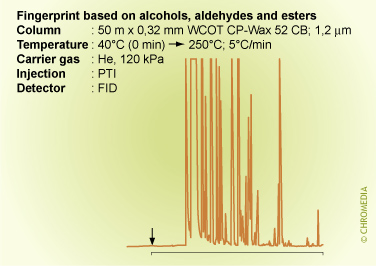
It is possible that we are not interested in the identity of the individual sample components, but in the identity of the complete mixture. For instance of petrochemical fractions, vegetable oils, foodstuff, etc. If a lot of chromatographic peaks are present from a complex mixture, a so-called 'fingerprint' can be produced. A pattern recognition profile can be made by means of a computer program which determines the identification (origin) of the sample unambiguously. It is required however that representative reference samples are available. In that case nothing can be concluded about the identity of the individual components, but conclusions can be drawn about the identity or purity of the sample.
Additional Methods
If the identity of the compound is not known and also if an absolutely positive identification is necessary, identification by chromatographic methods only is not acceptable. This is not a shortcoming of chromatography but inherent to chromatography, it is after all a separation technique and not an identification technique. A more specific column or a specific detector can help. It may be expected from a component which is monitored by e.g. a electron capture detector that it actually has electronegative properties and thus contains a certain constituent. As more data become available, the reliability of the identification increases. A similar sample analyzed on an entirely different chromatographic system will produce an entirely different chromatogram. With the aid of adequate chemical knowledge about interactions, more information can be obtained about the possible identity.
GC-Mass Spectrometry
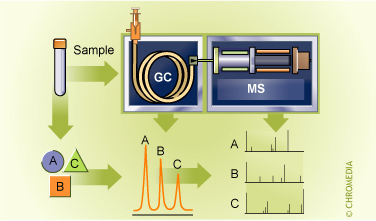
Unambiguous identification or verification can be carried out by using a spectroscopic method which actually provides information about the molecular structure. Nowadays GC analysis can be combined with IR or NMR , but the best results can be obtained by a mass spectrometer (MS). In such a combination the mass spectrometer can be regarded as a very sophisticated and specialized detector for the gas chromatograph while the gas chromatograph can be seen as a very sophisticated and specialized injector for the mass spectrometer. Price and the handling of these complex systems have now come within the scope of routine laboratories.

