Plate height equations
- Page ID
- 61083
Nico Vonk, Avans+, Breda, The Netherlands
Abstract
The efficiency of a column os affected by a number of variables:
- Column length
- Column diameter(open-tubular columns)
- Particle size (packed columns)
- Packing and coating quality
- Linear velocity (flow)
- Instrument quality (dead, void or hold-up volume)
- Retention factor k
Various equations have been developed to describe the influence of these variables on the column efficiency:
- The Van Deemter equation for packed GC columns
- The Golay equation for capillary columns
Level: Basic
Packed Columns: van Deemter
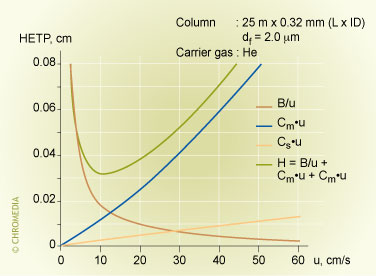
A plate height equation expresses the correlation between plate height and carrier gas velocity. It is called the van Deemter equation or H/u curve. Increasing the carrier gas velocity will initially lead to a sharp decrease of the plate height. The plate height will increase again after a minimum value has been obtained.
van Deemter H/u Curve
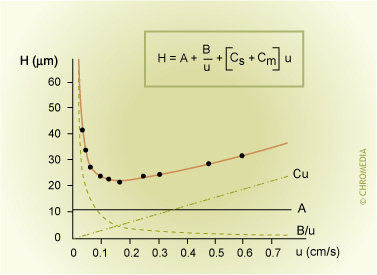
Answer to Phil's Tips question: Molecules will disperse (diffuse, spread) in the gas phase even if flow is zero. So the ratio of spreading to retention time in this case will be high (poor efficiency) when flow is low. Why is efficiency poor at high flow? Although molecules will not have much time to spread in the carrier phase, we have to think of what happens when molecules in the high speed carrier gas are physically moved down the column when other molecules are dissolved in the stationary phase (and so do not move down the column). If these molecules are left behind or retained in the stationary phase, then this corresponds to a broadening effect for the total band of molecules.
Peak broadening is governed by kinetic processes in the column such as molecular dispersion, diffusion and slow mass transfer. Identical molecules are dispersed in the column due to probability processes. We can consider three contributions to peak broadening:
- A-term: eddy diffusion: The column packing consists of particles with flow channels in between. Due to the difference in packing and particle shape, the speed of the mobile phase in the various flow channels differs and analyte molecules travel along different flow paths through the cannnels.
- B-term: longitudinal diffusion: Molecules traverse the column under influence of the flowing mobile phase. Due to molecular diffusion slight dispersions of the mean flow rate will be the result.
- C-term: resistance against mass transfer. A chromatographic system is in dynamic equilibrium. As the mobile phase is moving continuously, the system has to restore this equilibrium continuously. Since it takes some time to restore equilibrium (resistance to mass transfer), the concentration profiles of sample components between mobile and stationary phase are always slightly shifted. This results in additional peak broadening.
The various parameters that influence the overall peak width can be expressed in three terms:
\[H=A+\dfrac{B}{u}+C \times u\]
H = HETP (plate height)
A = eddy diffusion term
B = longitudinal diffusion term
u = linear gas velocity
C = resistance to mass transfer coefficient
Capillary columns: Golay equation
Capillary columns have a number of important advantages compared to packed columns. By far the most important characteristic of a capillary column is its very great separation power (resolution) due to its high efficiency. Capillary GC produces chromatographic peaks that are much narrower than peaks obtained from packed column GC. The illustration shows the difference in plate height between packed and capillary column. Capillary columns do not contain any column packing. Therefore there is no contribution to peak broadening from eddy diffusion.
Efficiency Packed vs Capillary Column
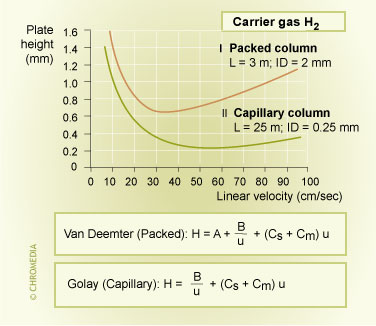
Peak broadening in a capillary column is mostly determined by the C-term, which is much smaller than in packed columns. Peak broadening in a packed column above the optimum gas velocity is caused by eddy diffusion (A-term) and the resistance to mass transfer (C-term).
Because the process is continuous, the band of molecules will travel down the column as a complete distribution of molecules. But the band will be kept ‘tighter’ together if there is fast equilibrium across the phase boundary between carrier and stationary phases. We want the molecules to get to the stationary phase fast, and then quickly equilibrate into, then out of, the stationary phase. Thus a high DM is favourable, and also a thin film column stationary phase is desirable.
Aromatics on a capillary GC column
Resistance to mass transfer (in particular the contribution of the Cm term) in packed columns is partly the result of the column stationary phase (in the Van Deemter equation by the presence of the particle size contribution dp). Therefore, in the absence of packing particles, peak broadening should be much smaller. This will have a positive effect on the efficiency of a capillary column.
The Golay equation describes plate height and peak broadening for capillary columns:
\[H=\dfrac{B}{u}+\left[C_m + C_s\right]u\]
Or more precise, with all parameters included (any stationary phase contribution is neglected):
\[H=\dfrac{2D_m}{u}+\left[\dfrac{1+6k+11k^2}{96D_m}\right]d_c^2 u\]
Factors that affect the plate height are:
- Diffusion coefficient in the mobile phase
- Retention Factor
- Column diameter
- Linear velocity
Narrow bore thin film columns therefore produce very small plate heights. More importantly, such columns can be operated at much higher gas velocities than wider columns. This is shown by the H-U curve resulting from the Golay equation for the optimum linear velocity. This means that a combination of high carrier gas velocity and the use of shorter columns can generate the same plate number as a longer wide-bore column, resulting in a much higher speed of analysis (short analysis time).
The lowest plate heights are obtained for thin-film columns. Thick-film columns have higher plate heights and hence lower plate numbers and efficiencies.
'H-u' Curve for Thin Film Columns
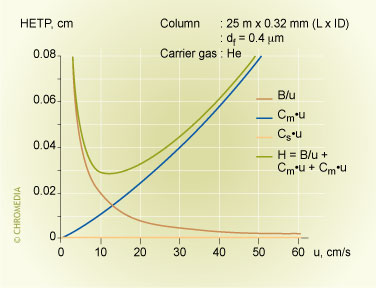
'H-u' Curve for Thick Film Columns
(NOTE: Narrow columns are often preferred over wide-bore columns. At some point however, pressure drop becomes the limiting factor. For any given maximum pressure drop, one can always generate more theoretical plates with wider columns than narrow columns, but at the expense of considerably more time as the columns must be very much longer).
The minimum plate height (the plate height at the optimum linear velocity) can be calculated from the Golay equation. For the first derivative (dH/du) is equal to zero. If we assume that the contribution of the Cs-term is negligible (this is allowed for thin film columns), the minimum plate height is:
\[H_{min}=r\sqrt{\dfrac{1+6k+11k^2}{3(1+k)^2}}\]
Above a value k > 5, the k-term will become approximately 2 and the above equation can be simplified to: Hmin = 2r = I.D.
In other words, the minimum plate height for thin film columns approximately corresponds to the internal diameter of the column!
The plate number of a column can be easily estimated from this simplified equation. A narrow bore column will always have a better efficiency (in terms of plate number) than a medium or wide-bore column!
Capillary columns have much higher efficiencies than packed columns mainly because of their higher permeability to the carrier gas. This means that much longer columns can be used at the same pressure drop. In addition, the plate height of a capillary column is smaller than that of a packed column due to the absence of the eddy diffusion term and smaller mass transfer terms in the Golay equation.
In general the maximum pressure drop available for GC is about 300 kPa, and this determines the maximum column length that can be used.
The internal diameter of a capillary column affects the Cm-term in the Golay equation resulting in a larger plate height at a larger diameter. The fact that there is no packing present does not mean that the efficiency of a capillary column will become independent of other column parameters. As with packed columns, the amount of stationary phase material, expressed in the film thickness, will affect the resulting efficiency.
The peak broadening is smallest with the smallest amount of stationary phase material.
The internal diameter of the column plays a part in the height of the Cm-term. The narrower the column, the higher the efficiency, but too narrow columns are difficult to operate due to the high pressure etc.
Eddy diffusion (A-term)
The A-term describes peak broadening due to multiple flow paths through a packed column bed. Eddy diffusion only occurs in packed columns. Packed columns are filled with particles and analyte molecules, therefore, cannot flow along a straight path through the column. Each analyte molecule follows a different flow path, which causes dispersion. Some molecules will travel more slowly and will lag behind the zone (peak) centre, others will follow shorter paths and are slightly ahead. This effect results in slight differences in elution times.
As can be seen from the van Deemter equation, eddy diffusion is independent of the gas velocity. The presence of stationary phase particles has a major influence on peak broadening. It is obvious that for open-tubular capillary columns the A-term is not relevant.
CHROMEDIA PROGRAM
Eddy diffusion is related to:
- Particle size dp: The larger the particles, the stronger the dispersion effect.
- Particle shape: With regularly shaped particles (spherical) the path length difference between the particles is smaller than with irregular particles. This is due to the fact that spherical particles can form a regularly packed bed more easily. Conversely, an irregular packed bed consists of flow channels of different shapes and diameter resulting in different mobile phase velocities.
- Pore structure/shape (pores in the particle)
- Quality of the column packing bed
- Wall effects (diameter, material, roughness)
Longitudinal diffusion is related to:
The B-term relates to molecular diffusion of analyte molecules in the mobile phase along the flow direction (column axis) of the mobile phase. Individual analyte molecules each have their own movement (diffusion velocity). This diffusion velocity is determined by the type of molecule, the nature of the mobile phase and the temperature, and is expressed quantitatively by the diffusion constants. These constants for the various carrier gases are greatest for lighter gases such as hydrogen and helium.
CHROMEDIA PROGRAM
Influences on B term:
- Linear velocity of the mobile phase
- Diffusion coefficient of component in mobile phase
- Temperature
- Type of carrier gas (density, diffusion)
Gas Velocity as Function of Internal Diameter
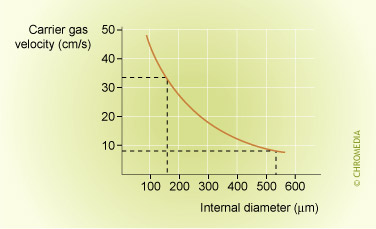
Longitudinal diffusion is the result of concentration differences in the mobile phase. In the center of the peak zone the concentration is at its maximum. The concentration before and after the peak zone is lower. This results in diffusion, both in the direction of the carrier gas flow as well as in the opposite direction.
This effect will be relatively great at long residence times in the column, which is the case at low flow rates. As the flow rate increases, this effect will contribute less to the total peak broadening. In practice, it is best to select flow rates that minimise the effect of longitudinal diffusion on column efficiency.
Resistance to mass transfer (C-term)
The C-term relates to the mass transfer of sample components between the stationary phase and the mobile phase. This term can be divided into a Cm-term and a Cs-term, describing the contributions to peak broadening in the mobile phase and in the stationary phase respectively. Analyte molecules are retained in a chromatographic system because they interact with the stationary phase. Since analyte molecules are present in the mobile phase, they have to move to and enter into the stationary phase in order to interact. Then they have to return to the mobile phase.
Because the linear velocity of the mobile phase is lower closer to the column wall (or the stationary phase particles) than in the center (or further away from the particles), the molecules experience different velocities. This results in peak broadening in the mobile phase. This phenomenon is described by the Cm term.
The Cm-term depends on:
- Particle size dp (packed columns)
- Internal diameter (capillary columns)
- Linear velocity u of the mobile phase
- Diffusion coefficient in the mobile phase
- Retention factor k
- Temperature
Resistance Against Mass Transfer
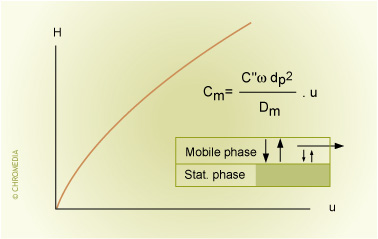
The overall C-term relates to the resistance to mass transfer that analyte molecules experience in both the mobile phase and the stationary phase.
Since the resistance to mass transfer in the mobile phase is not the same for all molecules of one type (it depends on the location in the column at a particular time and the distance they have to travel) this will also result in peak broadening of that component in the column.
Both C-terms can be described quantitatively by a number of parameters. Without discussing these in detail it can be concluded that peak broadening due to the Cm-term is smallest when:
- the stationary phase particles in the column are small or
- the column diameter is small in case of open-tubular columns(!)
- the diffusion coefficients (the molecular diffusion in the carrier gas)are large, This is the case for hydrogen or helium as carrier gas.
The Cs-term is determined by the amount of stationary phase (low is advantageous for the efficiency) and the extent of interaction of the sample on the phase (represented by the retention factor) and the distances the sample molecules have to travel.
Influences on CSterm:
- Amount of stationary phase (film thickness)
- Diffusion coefficient in stationary phase
- Coating efficiency (uniform film)
- Retention factor k
- Temperature
- Linear velocity u of the carrier gas
- Particle size (packed column)
Resistance Against Mass Transfer in Stationary Phase
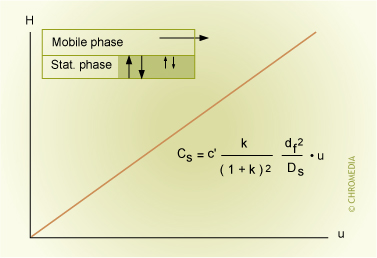
Small retention factors have a positive effect on the efficiency. Up to k = 5 the effect on peak broadening increases until a constant value.
In addition to the amount of stationary phase and the degree of interaction, the diffusion of the molecules in the stationary phase plays a role as well. The diffusion coefficient in the stationary phase is dependent on the type of sample, the type of stationary phase and the temperature. A large diffusion coefficient has a positive effect on the efficiency.
From a theoretical point of view, the peak broadening as a result of the resistance to transfer is proportional to the gas velocity; hence it will be largest at high flows. The H-u curve shows straight curves. In practice, however, the lines are slightly curved due to compressibility effects in the mobile phase.
The 'speed' of a Capillary Column
A very important property of capillary columns is that they are fast. Analysis times are significantly shorter compared to packed columns.
From the Golay equation, we can calculate that:
\[u_{opt}=\dfrac{D_m}{d_c}\sqrt{\dfrac{2}{f_g(k)}}\]
with:
\[f_g(h)=\dfrac{1+6k+11k^2}{96(1+k)^2}\]
The optimum gas velocity is inversely proportional to the column diameter.
Narrow-bore columns are faster than medium or wide-bore columns. A further advantage of capillary columns over packed columns is that the gas velocity and/or the column temperature can be increased quite easily if the resolution allows. This brings about an even bigger increase in analysis speed. Also, the same relationship shows that a lighter carrier gas such as hydrogen or helium gives faster analysis without a significant loss in resolution.

