Bonded-Phase Liquid Chromatography
- Page ID
- 71835
In our development of band broadening, we talked extensively about the C18 columns that are the most common in use today. We already mentioned that less common bonded phases including those with C8, C1, aminopropyl (C3H6NH2) and cyanopropyl (C3H6CN) groups are also commercially available. Prior to the introduction of the C18 columns, the normal way to do liquid chromatography involved the use of a polar stationary phase like silica gel and a non-polar mobile phase. When the C18 columns were first reported, they constituted a non-polar stationary phase, and so a polar mobile phase could be employed. Since this configuration of polarities of the phases was the reverse of what was normally done, the use of C18 columns became known as reversed-phase liquid chromatography. Even though today almost everyone uses these reversed-phase methods, the name has stuck.
We have already seen how the deactivation of the surface with the C18 groups has a very positive effect on the chromatographic efficiency. Another significant advantage of using C18 stationary phases is that aqueous-based mobile phases are used with them. Water is the ideal mobile phase for liquid chromatography because so many substances are soluble in water. Water is the solvent of the environment. Water is the primary solvent of living systems. Many pharmaceutical agents need to possess water solubility to enter the body. Since many organic compounds are not that soluble in water, organic pharmaceuticals that are not water-soluble usually have ionic groups incorporated into them to render them water-soluble.
It’s rare to use only water as the mobile phase with a C18 column. Instead, an organic modifier (methanol, acetonitrile, and tetrahydrofuran are the three most common) is added in some amount to the mobile phase. Compared to water, the chemical properties of the organic modifier are more like that of the C18 phase. The presence of the organic modifier therefore shortens the retention time compared to a mobile phase that was only water. Raising the concentration of the organic modifier will further shorten the retention time of almost all compounds. We mentioned the concept of a gradient elution earlier in the unit when we discussed the problem of having a limited peak capacity in a chromatogram. Remember the goal was to start with a high retention factor and systematically change the system so that the retention factor got shorter as the chromatogram progressed. One possible gradient procedure might be to start with a mobile phase that is 80:20 water-methanol and gradually change is to 20:80 water-methanol during the chromatogram. The increased concentration of methanol will reduce the retention factor of the later eluting components.
Experimentally, it is easy to carry out a gradient. Using two pump heads, one pumps the initial phase and the other the final phase. A special mixing chamber exists to adequately mix the output from the two pumps. During the chromatogram, a computer systematically slows down the pump rate of the initial phase and ramps up the pump rate of the final phase.
Another important variable with an aqueous mobile phase is the pH of the system. C18 silica gel phases are stable over the range of 2 to 8 (although 3 to 7 really represents a safer window). If the pH is too acidic, the surface C18 groups will be hydrolyzed off. If the pH is too basic, the silica gel itself will decompose. This pH range is one that can be used to change the nature of many acids and bases. For example, carboxylic acids at pH 3 might mostly be in their protonated (neutral) form, whereas at pH 7 they are likely to be in their deprotonated (anionic) form. Since we would expect the neutral form to have more solubility in a C18 phase then the anionic form, the retention time for most carboxylic acids would be longer at pH 3 than at pH 7. If we consider nitrogen bases, we note that at pH 3 they are likely to be in their protonated (cationic) form, whereas at pH 7 they are likely to be in their deprotonated (neutral) form. Organic bases therefore would have a longer retention time at pH 7 than at pH 3. You might also recollect how we used a similar process to distinguish acids and bases in an acid/base/neutral extraction procedure discussed at the beginning of this unit. This is the same behavior and rationale going in liquid chromatography. Another gradient procedure that could be used to advantage in liquid chromatography is a pH gradient.
There is some debate in the literature on liquid chromatography about the exact nature of the separation procedure (adsorption on a deactive surface versus partitioning), and whether stationary phase or mobile phase (solvophobic) effects are most important in determining retention properties. It actually turns out that either one usually leads to the same conclusion as to retention order, and for our purposes, thinking about which compound of a pair has a greater attraction for the stationary phase is sufficient to predict which will come out first and which second. We have already examined the two pairs below as a function of pH.
RCOOH RCOO–
pH = 3 (longer tR) pH = 7 (shorter tR)
R3NH+ R3N
pH = 3 (shorter tR) pH = 7 (longer tR)
How about a comparison of the retention order of pyridine and tert-butylpyridine on a reversed-phase column? If you thought that the presence of the tert-butyl group on the pyridine would make that compound more attractive to the C18 groups, thereby causing it to have the longer retention time of the two, you are correct. In fact, in all likelihood, there is some preference of the association of these two compounds with a C18 phase as shown in Figure 68. The non-polar portion of the molecule is likely oriented toward the C18 phase, whereas the polar nitrogen atom is oriented toward the mobile phase.
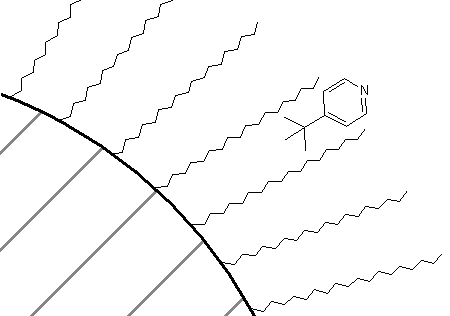
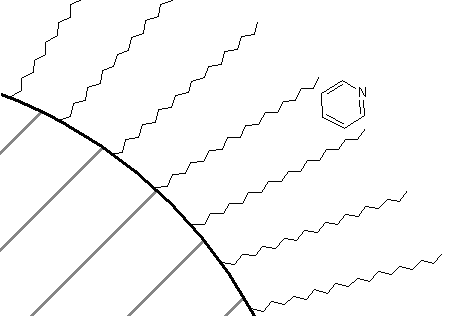
Lastly, picking up on another technique we learned with regards to liquid-liquid extractions, we could ask how we might lengthen the retention time of organic cations on a C18 column. For example, there are a series of neurotransmitters such as dopamine that are based on aromatic amine derivatives.
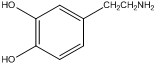
dopamine
Dopamine and other similar derivatives may well be protonated under the separation conditions, and because of the protonation, have short retention times that do not facilitate the separation of the similar analogues. Can you think of a way to lengthen the retention time that does not involve adjustment of the pH? A way to do this is to add a “greasy” anion such as heptylsulfonate to the mobile phase (note, since this is added to the mobile phase the anion is continuously pumped through the system).
\[\mathrm{C_7H_{15}SO_3^- = heptylsulfonate}\]
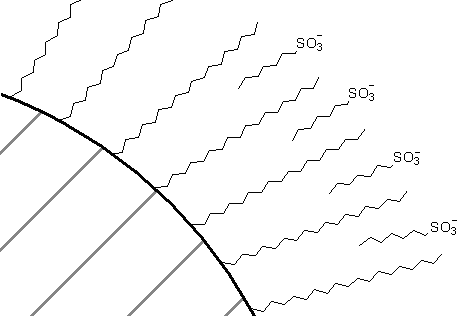
One way to think of this is that, just like in liquid-liquid extraction, the sulfonate anion can form an ion pair with the cation and the ion pair would have more solubility in a C18 phase than the cation by itself. An alternative way to view this is that some of the sulfonate salt distributes into the C18 phase as shown in Figure 69. This serves to create a pseudo ion exchange material that the organic cations are attracted to. Detailed studies have shown that the latter mechanism (pseudo ion exchange resin) is the one that actually operates.
One other important category of bonded phases involves the attachment of optically pure groups to silica gel. The compound dinitrobenzoyl-L-leucine is one example of such a phase. These types of phases are used for the separation of enantiomers.
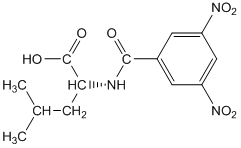
Dinitrobenzoyl-L-leucine
The distinction of enantiomers is analogous to the distinction that would occur if you considered putting your feet into only one of your shoes. If you put your left foot into your left shoe, it feels fine. If you put your right foot into your left shoe, it does not fit quite as well, and even in the dark, you would notice that something isn’t right. If we expressed this as a reaction with an equilibrium constant, we could say that the association of your left foot with your left shoe is higher than the association of your right foot with your left shoe. If we had bonded the left shoes to silica gel, your right foot would elute faster from the column, your left foot slower. The compound pictured above has a number of groups that can form intermolecular interactions with enantiomeric solutes. The amide functionality is a site with dipoles and the potential for hydrogen bonding. The electron-deficient dinitrobenzoyl ring can undergo what is known as pi-stacking with electron-rich aromatic rings. The isobutyl group of the leucine provides steric hindrance. Other chiral liquid chromatographic phases are designed to have similar groups that provide sites for intermolecular attraction, π-stacking, and steric constraints.


