2.2: How is the internal standard used to quantify the concentration of my analyte?
- Page ID
- 77771
If an NMR spectrum is measured with care, the integrated intensity of a resonance due to the analyte nuclei is directly proportional to its molar concentration and to the number of nuclei that give rise to that resonance.
\[\mathrm{\dfrac{Integral\: Area}{Number\: of\: Nuclei} ∝ Concentration} \label{E1}\]
For example the 1H NMR resonance of a methyl group would have 3 times the intensity of a peak resulting from a single proton. In the spectrum below for isopropanol, the 2 methyl groups give rise to a resonance at 1.45 ppm that is 6 times greater than the integrated intensity of the CH resonance at 3.99 ppm. Since this spectrum was measured in D2O solution, only the resonances of the carbon-bound protons were detected. The OH proton of isopropanol is in fast exchange with the residual water (HOD) resonance at 4.78 ppm, therefore a separate resonance is not observed for this proton.
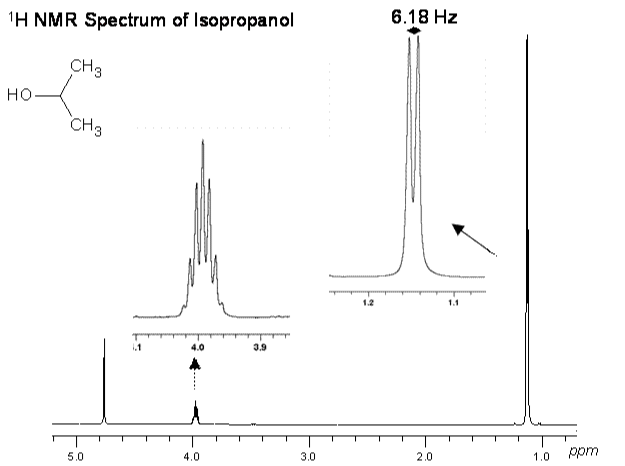
In this example we compared the relative integrals of the proton resonances of isopropanol. This information can be very useful for structure elucidation. If we instead compare the integral of an analyte resonance to that of a standard compound of known concentration, we can determine the analyte concentration.
\[\mathrm{Analyte\: Concentration = \dfrac{Normalized\: Area\: Analyte \times Standard\: Concentration}{Normalized\: Area\: Standard}} \label{E2}\]
The direct proportionality of the analytical response and molar concentration is a major advantage of NMR over other spectroscopic measurements for quantitative analysis. For example with UV-visible spectroscopy measurements based the Beer-Lambert Law, absorbance can be related to concentration only if a response factor can be determined for the analyte. The response factor, called the molar absorptivity in UV-visible spectroscopy, is different for each molecule therefore, we must be able to look up the absorptivity or have access to a pure standard of each compound of interest so that a calibration curve can be prepared. With NMR we have a wide choice of standard compounds and a single standard can be used to quantify many components of the same solution.
Question 1. A quantitative NMR experiment is performed to quantify the amount of isopropyl alcohol in a D2O solution. Sodium maleate (0.01021 M) is used as an internal standard. The integral obtained for the maleate resonance is 46.978. The isopropanol doublet at 1.45 ppm produces an integral of 104.43. What would you predict for the integral of the isopropanol CH resonance as 3.99 ppm. What is the concentration of isopropanol in this solution?


