Flame Emission Spectroscopy
- Page ID
- 75259
Flame atomic emission spectroscopy (FAES) is a classical method which has been largely displaced by plasma spectroscopies. Plasmas produce higher atomization ratios, but the theory is similar in both flame and the plasmas. FAES is the classical method used as plasmas have taken over as the preferred method due to the higher atomization ratios that occur. Using the flame could be advantageous in a Group I or Group II elemental analysis since less ionization will occur at lower temperatures (compared to a plasma). It is typically not used often, unless sensitivity and cost are possible issues.
Below is a very simple schematic for a laminar flow burner. The Primary Combustion Zone is where the initial decomposition occurs and molecular fragments are observed. The Interzonal Region is the hottest part of the flame and atomic fragment are observed. The Secondary Combustion Zone is cooler overall and a conversion is seen from atoms back to stable molecules and oxides.
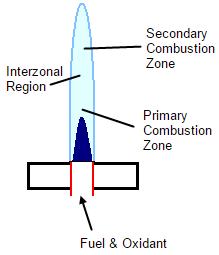
Fuel (usually acetylene) and air are added mixed with a nebulizer mist. This mixture is then introduced into the flame.
The advantages to the Laminar flow burner are that it is cheap, simple, relatively stable and can operate at lower temperatures. An issue that complicates flame emission just as it complicates plasma emission is self reversal.
The sketch below shows a flame in an FAES system. What is going to happen to an excited atom (A*) in this flame? It is going to give off radiation in any direction. But where is the detector, which is in this case just an eye. What is in between the A* and the detector? There is an analyte atom in the ground state. Can this ground state atom absorb the photon? Why?



