Amino Acids
- Page ID
- 302706
It is worth highlighting the acid-base properties of amino acids, since these are so important in biochemistry. The structure of the amino acid alanine, as you would typically see if written, is shown below.
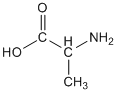
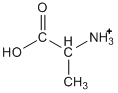
If you look in our chart of pKa values, you would find that two values are given for alanine (pKa1 = 2.34; pKa2 = 9.69), which might surprise you at first. As you look at the structure, remember that the -COOH group is an acid, but also that an amine group (NH2) is a base. There are two pKa values because we can protonate the amine group, as shown below.
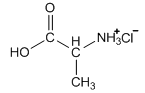
Usually the protonated form is prepared by reaction with hydrogen chloride, so instead of referring to the alaninium ion, we would call it alanine hydrochloride.
One last thing to consider about the neutral amino acid. If we have a substance that has an acid (-COOH) and base (-NH2) within the same molecule, we could ask whether this could undergo an internal acid-base neutralization reaction (realize that we would have many of these molecules in solution so we could also view the acid and base functionalities of different alanine molecules neutralizing each other). It turns out that this actually occurs with amino acids in water, leading to an alanine species with two charges that is called a zwitterion.
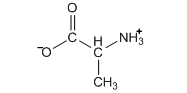.png?revision=1)
Note that the zwitterion still has a net neutral charge, so we do not need to distinguish whether its written form is neutral or zwitterionic in equilibrium calculations.
Contributors and Attributions
- Thomas Wenzel, Bates College (twenzel@bates.edu)
- Sourced from the Analytical Sciences Digital Library


