Gravimetric Analysis (Heiss)
- Page ID
- 281944
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
The amount of iron in a dietary supplement tablet can be determined by converting the soluble iron to solid Fe2O3 as outlined in the steps below:

Tablets containing iron(II) fumerate (Fe2+C4H2O42-) and inert binders are mixed with 150 mL of 0.100 M HCl to dissolve the Fe2+. Mixture is filtered to remove insoluble binder.
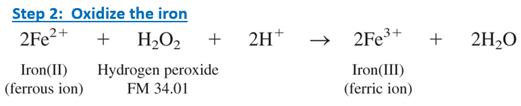
Iron(II) in the clear liquid is oxidized to iron(III) with excess hydrogen peroxide.
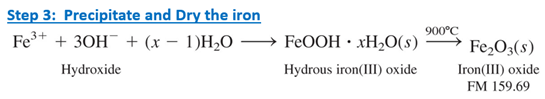
Ammonium hydroxide is added to precipitate hydrous iron(III) oxide, which is a gel. The gel is filtered and heated in a furnace to convert it to pure solid Fe2O3.
- Suppose each of these daily supplement tablets provides ~15mg of iron. How many tablets should we analyze to provide 0.25g of Fe2O3 product?
- What mass of 3.0 wt% H2O2 solution is required to provide a 50% excess of reagent for the reaction below with 12 iron tablets?
- The mass of Fe2O3 collected from 12 tablets was 0.277g. What was the average mass of iron per dietary tablet?
Contributors and Attributions
- Dr. Elise M. Heiss, Kings College (EliseHeiss@kings.edu)
- Adapted from: Harris, D. C. Quantitative Chemical Analysis; W. H. Freeman and Company: New York, 2016; Ch. 1
- Sourced from the Analytical Sciences Digital Library


