Gas Chromatography: End of Unit Problem Solving
- Page ID
- 284464
Modeling Activity – Kathryn Riley, Swarthmore College
Context: I used this activity at the conclusion of our class discussion of GC, which occurred late in the semester. At this stage, students had already covered statistics, calibration, sampling, sample preparation, electrochemistry, spectroscopy, and the fundamental principles of chromatography. The overall purpose of this activity was to give students more guided practice in developing a top-to-bottom analytical method.
Learning Objectives for GC Separation Scenarios:
- Label an internal standards calibration curve.
- List the requirements of an internal standard.
- Predict the elution order of analytes.
- Develop a sample preparation strategy.
- Propose an internal standard and GC stationary phase for an analysis.
- Choose an appropriate detector for a GC analysis.
The following two papers were used in designing this activity.
Scenario 1: https://pubs.acs.org/doi/abs/10.1021/ed078p1231
Scenario 2: https://link.springer.com/article/10.1007/s00216-018-01569-1?shared-article-renderer
Gas Chromatography Separation Scenarios
- Chlorination processes are often used to disinfect drinking water and swimming pools. However, chlorine species can react with natural organic matter to produce by-products that can have toxic health effects. One class of these disinfection by-products is trihalomethanes (THMs). THMs form through reaction of chlorine with organic substances in the water, including organic molecules produced by humans (e.g., urine, sweat, hair, flakes of skin).
You are developing a GC-MS method to detect and quantify the presence of THMs in the Ware Pool water at Swarthmore College. The THMs you wish to analyze, as well as the internal standard (IS) you will use for calibration, are listed in the table below along with their boiling points.
THM
Chemical Formula
b.p. (°C)
chloroform
CHCl3
142
bromoform
CHBr3
300
bromodichloromethane
CHBrCl2
194
dibromochloromethane
CHBr2Cl
120
IS: 1,2,3-trichloropropane
C2H5Cl3
313
- Below is a plot of your internal standards calibration curve. Label the axes and annotate the plot to show how you will determine the concentration of the THMs in pool water.
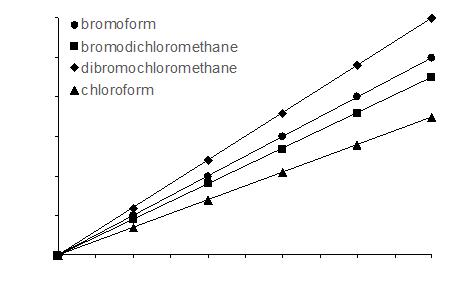
- For which analyte does your analyte have the highest sensitivity? The lowest?
- Gas chromatography samples are typically prepared in organic solvents that will easily evaporate in the sample injection/vaporization chamber. However, your samples are in aqueous solution. List two ways that you can prepare your pool water samples. Then discuss how you would prepare an appropriate blank and your calibration standards.
- Assuming the liquid stationary phase you are using for the separation is a good match for all of the compounds you wish to analyze, what is the predicted elution order? Label the peaks on the chromatogram.
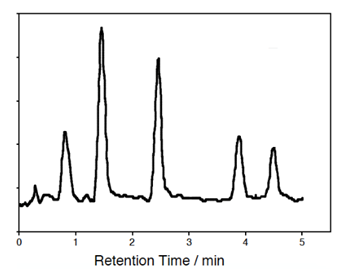
- List three ways you could confirm these peak identities.
- Suggest an alternative detection strategy that would be sensitive for THMs.
- Below is a plot of your internal standards calibration curve. Label the axes and annotate the plot to show how you will determine the concentration of the THMs in pool water.
- Polycyclic aromatic hydrocarbons (PAHs) are a large component of smoke and result from the incomplete combustion of organic materials. Some PAHs are carcinogenic and have been linked to human cancers. For the general public, exposure to PAHs often results from air pollution or tobacco smoke, but for firefighters, exposure to PAHs is an occupational hazard. In humans, PAHs undergo biotransformation and are excreted in urine as hydroxylated metabolites. Urinary 1-hydroxypyrene is widely used for biomonitoring human exposures to PAHs, but the determination of other metabolites (like those in the table below) can provide a more accurate assessment of total chemical exposures.
Chemical Name
Chemical Structure
b.p. (°C)
1-hydroxypyrene

298
2-hydroxynapthalene
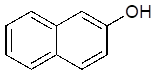
285
3-hydroxyphenanthrene
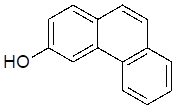
405
9-hydroxyfluorene

368
You wish to measure the PAH exposure of firefighters who were involved in putting out the historic “Camp Fire” in 2018 – a wildfire that destroyed over 150,000 acres of land, 18,000 homes, and resulted in at least 85 civilian fatalities, with a total cost of $16.5 billion. The fire reached containment after 17 days of sustained effort, so firefighters involved in controlling the fire had significant and prolonged exposure to PAHs. The questions below will help you develop a GC method for quantifying the PAH exposure of firefighters by measuring the concentrations of the hydroxylated metabolites.
- Human urine is comprised of approximately 95% water, as well as a number of inorganic salts and organic compounds, like proteins, hormones, and metabolites. The exact composition varies from person-to-person depending on their body chemistry and diet. You were able to collect mid-stream urine samples from 50 firefighters (40 male, 10 female) involved in the “Camp Fire”. Describe how you will prepare the urine samples so that they can be directly injeced into a GC instrument.
- You plan to use the internal standards method to prepare your calibration curve. List the requirements of an internal standard.
- Propose an internal standard for the analysis by drawing a chemical structure. Would you expect this molecule to have a different elution time than your analytes? Why?
- You plan to carry out your GC separation on a fused-silica capillary coated with a liquid stationary phase. From the list below, choose an appropriate stationary phase for the separation. Justify your choice.
polydimethyl siloxane
5% phenyl-polydimethyl siloxane
50% phenyl-polydimethyl siloxane
50% trifluoropropyl-polydimethyl siloxane
polyethylene glycol
50% cyanopropyl-polydimethyl siloxane
- Choose an appropriate detector for the unambiguous determination of the metabolites. Justify your choice.
- Human urine is comprised of approximately 95% water, as well as a number of inorganic salts and organic compounds, like proteins, hormones, and metabolites. The exact composition varies from person-to-person depending on their body chemistry and diet. You were able to collect mid-stream urine samples from 50 firefighters (40 male, 10 female) involved in the “Camp Fire”. Describe how you will prepare the urine samples so that they can be directly injeced into a GC instrument.
Contributors and Attributions
- Kathryn Riley, Swarthmore College (kriley1@swarthmore.edu)
- Sourced from the Analytical Sciences Digital Library


