EDTA Chelation and Titrations
- Page ID
- 282854
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
Reading: Chapter 13 (sections 1-6), Exploring Chemical Analysis 5th ed., D. Harris
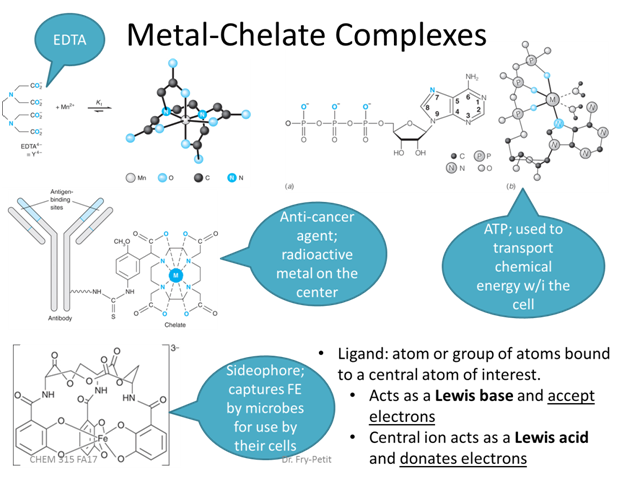
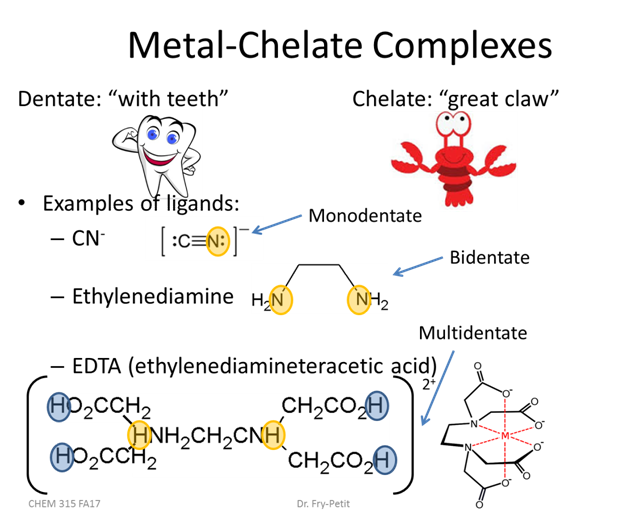
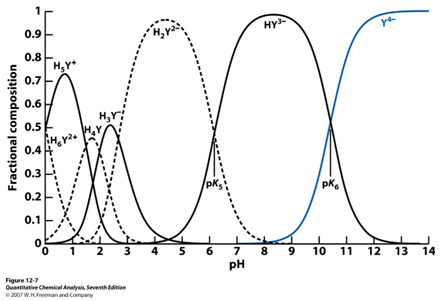
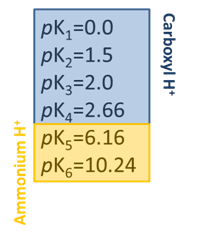
- Practically what does this fractional composition diagram mean for EDTA titrations?
STOP

- Calculate the concentration of free Ca2+ in a solution of 0.10 M CaY2- at pH 10.00 and at pH 6.00 with the conditional formation constant.
- What is the rxn we are thinking about?
- Use an ice table to determine the amount of free (uncomplexed) Ca2+
- What is the rxn we are thinking about?
STOP
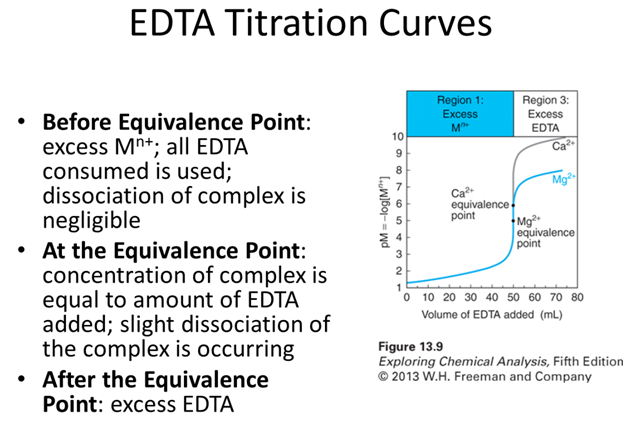
- Calculate the pCa2+ vs. mL EDTA for the reaction of 50.0 mL of 0.0400 M Ca2+, buffered to pH 10.00, with 0.0800 M EDTA at the following volumes.
- Before the equivalence point: 5.0 mL EDTA
STOP
- At the equivalence point
STOP
- After the equivalence point: 26.0 mL
- Before the equivalence point: 5.0 mL EDTA
STOP
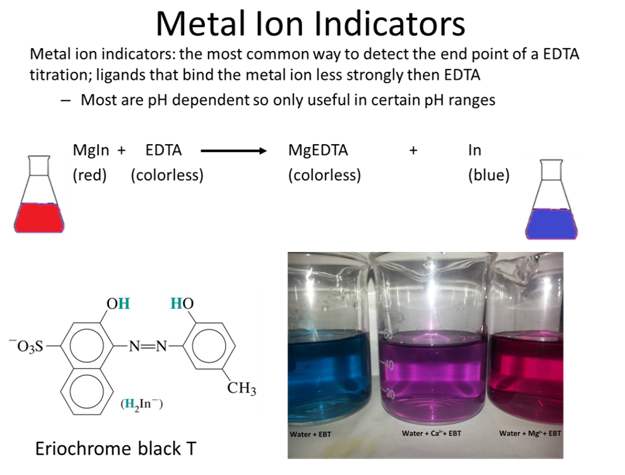
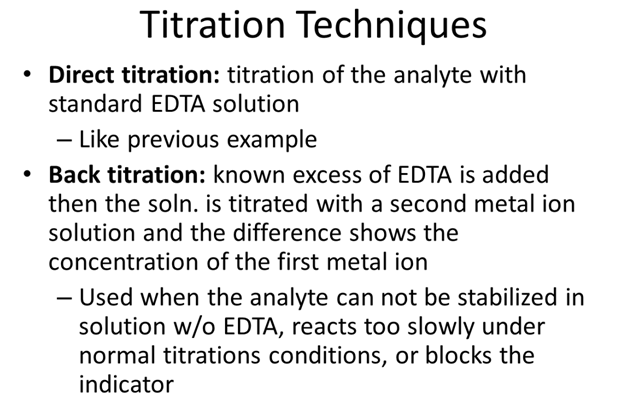
- Ni2+ can be analyzed by back titration, using standard Zn2+ at pH 5.5 w/ xylenol orange indicator. A solution containing 25.00 mL of Ni2+ in dilute HCl is treated w/ 25.00 mL of 0.05283 M Na2EDTA. Titration w/ 0.02299 M Zn2+ requires 17.61 mL to reach the red end point. What is the molarity of the Ni2+?
STOP
Contributors and Attributions
- Allyson Fry-Petit, California State University Fullerton (afry@fullerton.edu)
- Sourced from the Analytical Sciences Digital Library


