In-class Questions: Molecular Luminescence
- Page ID
- 105714
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
Energy level diagrams for organic molecules
- Draw an energy level diagram for a typical organic compound with \(\pi\) and \(\pi\) * orbitals and indicate which orbitals are filled and which are empty.
- Now consider the electron spin possibilities for the ground and excited state. Are there different possible ways to orient the spins (if so, these represent different spin states).
- Do you think these different spin states have different energies?
- Which one do you expect to be lower in energy?
- If the spin state is defined as (2S + 1) where S represents the total electronic spin for the system, try to come up with names for the ground and possible excited states for the system that are based on their spin state.
- Draw a diagram of the energy levels for such a molecule. Draw arrows for the possible transitions that could occur for the molecule.
- What do you expect for the lifetime of an electron in the T1 state?
- Why is phosphorescence emission weak in most substances?
- Which transition (\(\pi\)*-\(\pi\) or \(\pi\)*-n) would have a higher fluorescent intensity? Justify your answer.
Instrumental considerations for luminescence measurements
- What would constitute the basic instrumental design of a fluorescence spectrophotometer?
- What would be the difference between an excitation and emission spectrum in fluorescence spectroscopy?
- Draw representative examples of the excitation and emission spectrum for a molecule.
- Describe a way to measure the phosphorescence spectrum of a species that is not compromised by the presence of any fluorescence emission.
- If performing quantitative analysis in fluorescence spectroscopy, which wavelengths would you select from the spectra you drew in the problem above?
- Which method is more sensitive, absorption or fluorescence spectroscopy?
Variables that influence fluorescence measurements
- What variables influence fluorescence measurements? For each variable, describe its relationship to the intensity of fluorescence emission.
- Consider the reaction shown below for the dissociation of 2-naphthol. This reaction may be either slow (slow exchange) or fast (fast exchange) on the time scale of fluorescence spectroscopy. Draw the series of spectra that would result for an initial concentration of 2-naphthol of 10-6 M if the pH was adjusted to 2, 8.5, 9.5, 10.5, and 13 and slow exchange occurred. Draw the spectra at the same pH when the exchange rate is fast.
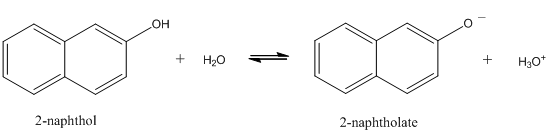
- Devise a procedure that might allow you to determine the pKa of a weak acid such as 2-naphthol.
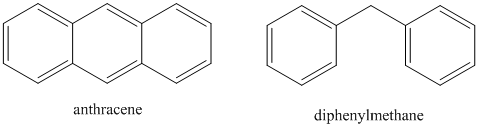
- Which compound will have a higher quantum yield: anthracene or diphenylmethane?