7.3: Coulometry
- Page ID
- 81969
Coulometry is similar to electrogravimetry in that a constant potential is applied that is sufficient to carry out a particular electrochemical reaction. In this case, instead of plating out a metal and measuring its weight, the current generated through the electrochemical reaction is measured as a function of time. As with electrogravimetry, it is essential that all of the species in solution undergo the electrochemical reaction. To accomplish this, the potential is typically applied to the solution for 30-60 minutes, an electrode with a large area is used and the solution is stirred. The charge (Q) can be related to the number of electrons using Faraday’s Constant, which can then be related to the moles of substance being measured. We can use the reduction of Cd2+ to cadmium metal to examine the technique of coulometry.
Draw the plot you would obtain for current (y-axis) versus time (x-axis) if you applied a constant potential high enough to carry out the reduction of Cd2+ to cadmium metal.
Solution
Just like in electrogravimetry, the concentration of Cd2+ is high at the beginning of the analysis and diminishes as it is reduced to cadmium metal. This means that the current starts out high and will diminish with time. That leads to the plot in Figure 11.
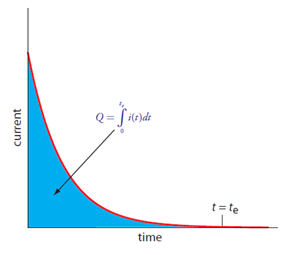
Figure 11. Plot of current versus time in a coulometric analysis. (Figure from Analytical Chemistry 2.0, David Harvey, community.asdlib.org/activele...line-textbook/).
How would you relate the outcome of the plot in Figure 11 to the concentration of Cd2+ in the sample?
Solution
What is important to determine here is the total amount of charge that flowed as that can be related using Faraday’s constant to the total number of electrons that were used in reducing Cd2+. The total number of electrons can be related to the total moles of cadmium in solution. Determining the total charge would involve integrating the area under the curve in Figure 11.
Advantages of Coulometry over Electrogravimetry
There are several advantages that coulometry has over electrogravimetry.
Since coulometry measures the charge needed to complete the electrochemical reaction instead of the weight of the substance plated out, reactions in which both species are water soluble can be examined (e.g., the half reaction \(\ce{Fe^{3+}(aq) + e^{–} -> Fe^{2+}(aq)}\)).
Electrogravimetry is only useful for reduction processes involving the plating of a metal. Coulometry can be used in either a reduction or oxidation mode, increasing its versatility. The direction in which the electrons flow will be different depending on whether the reaction involves a reduction or oxidation, but that is not a hindrance to measuring the current and obtaining a plot like that in Figure 11.
One last advantage is that coulometry is more sensitive than electrogravimetry. The sensitivity and detection limits in electrogravimetry are limited by the minimum weight that can be measured on a balance – analytical balances typically measure down to 0.0001 gram but a much higher weight is needed for suitable accuracy and precision. We have the ability to measure very small quantifies of current and can accurately measure time.


