Origins of Lithia Water
- Page ID
- 199370
Q8. If the Lithia spring water was to leach ions from limestone deposits to form soda springs, what primary cations and anions would you expect to have dissolved in the spring water due to such leaching action?
Calcium ions and carbonate ions.
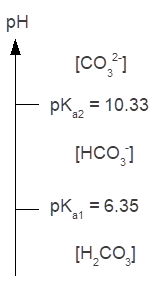
The pH of Lithia water is typically 6.4, and based on the ladder diagram for the carbonic acid system, the predominant form of carbonic acid is the bicarbonate ion and carbonic acid. Since the pH of Lithia water is slightly greater than pKa1, there is slightly more bicarbonate ion present than carbonic acid.