Chemical Equilibria and Sample Preparation
- Page ID
- 195414
Learning Outcomes
The purpose of this assignment is to predict how solute concentrations are controlled by chemical equilibria, understand the chemistry involved with sample preparation, and the extent to which sample preparation is necessary prior to analyte determinations.
At the end of this assignment, students will be able to:
- Assess the effect of pH and redox chemistry on solubility equilibria in Lithia water
- Develop appropriate strategies for sample preparation prior to analysis of Lithia water
Redox Chemistry
In the Major Inorganic Constituents module, you learned about the main ionic solutes present in Lithia water, however, dissolved gases are also present. Of these gases, diatomic oxygen (O2) can act as an oxidizing agent:
\[\ce{O2 (g) + 4 H+ (aq) + 4 e- \rightleftharpoons 2 H2O (l)} \hspace{25px} \mathrm{E^o = 1.23\: V}\nonumber\]
This is a very favorable reduction half-reaction. Dissolved species that can be oxidized by dissolved oxygen need to have valence electrons available. Therefore, one can initially evaluate whether a redox reaction should occur by determining whether a dissolved chemical species has any available valence electrons that can reduce oxygen to water.
Q1. Which of the cationic solutes in Lithia water are main group ions? Which of the cationic solutes are transition metal ions? Evaluate whether main group and transition metal cations have lost all of their valence electrons. Can main group and/or transition metal cations potentially be oxidized by dissolved oxygen?
In the Analytical Chemistry 2.0 text (http://www.asdlib.org/onlineArticles/ecourseware/Analytical Chemistry 2.0/Text_Files_files/AnalChem2.0.pdf), the author introduces the concept of ladder diagrams as a vehicle for evaluating whether a redox reaction can take place based on reduction potentials. A list of standard reduction potentials is available in Analytical Chemistry 2.0. The ladder diagram in Figure 1 illustrates whether dissolved oxygen could oxidize a transition metal ion, such as tin (II).
\[\ce{Sn^4+ (aq) + 2e- \rightleftharpoons Sn^2+ (aq)} \hspace{25px}\mathrm{E^o = +0.13\: V}\nonumber\]
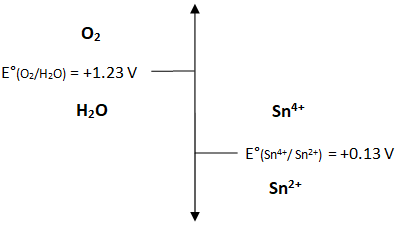
Figure 1. A ladder diagram for the redox couple of dissolved oxygen and divalent tin under standard conditions.
The overlap in the ladder diagram with dissolved oxygen and tin (IV) indicates the ability of these two species to coexist in solution, highlighting tin (II) as a reducing agent.
A more complete evaluation of such redox chemistry involves the use of the Nernst equation since it is rare to encounter redox chemistry in the environment under standard conditions (25 °C, 1 M concentration for each species in solution or 1 atm pressure for gas-phase species). The Nernst equation is shown in eq 1:
\[E= E^o - \frac{RT}{nF} \ln K\tag{1}\]
In eq 1, Eo is the half-reaction potential (V) under standard conditions, E is the half-reaction potential (V) under non-standard conditions, K is the equilibrium constant for a given reaction, R is the gas constant (8.314 J K-1 mol-1), T is the temperature (K), n is the number of electrons transferred and F is Faraday’s constant (96,485 C mol-1). Combining constants at 298 K and converting from natural to base 10 logarithm yields eq 2:
\[E= E^o - \frac{0.05915}{n} \log K \tag{2}\]
For the reduction half-reaction of oxygen to water in eq 3:
\[K= \dfrac{1}{[O_2 ] [H^+ ]^4 }\tag{3}\]
Assuming a dissolved oxygen concentration of 10 mg/L (3.1 x 10-4 M) and a pH of 6.0:
\[E=1.23V- \dfrac{0.05915}{4} \log \dfrac{1}{[3.1\times10^{-4} M] [1.0\times10^{-6} M]^4 }=0.823V\nonumber\]
An updated ladder diagram under non-standard conditions is shown in Figure 2.
Figure 2. Ladder diagram for the redox couple of dissolved oxygen and divalent tin under non-standard conditions described in the text above.
Even under these conditions, dissolved oxygen can be a potent oxidizing agent.
Q2. Using the standard reduction tables in Analytical Chemistry 2.0, draw a ladder diagram for the redox couple of dissolved oxygen and Fe3+/2+ under standard conditions. Predict the predominant oxidation state for iron under these conditions.
Q3. Using the standard reduction tables in Analytical Chemistry 2.0, draw a ladder diagram for the redox couple of dissolved oxygen and Fe3+/2+ assuming the following non-standard conditions: assume that the dissolved oxygen concentration is 10 mg L-1, the total iron concentration is 10 mg L-1, and that the initial ratio of [Fe2+]/[Fe3+] is 1000. Predict the predominant oxidation state for iron under these conditions and compare the result to your answer in question 2.
Q4. Refer to the plaque shown in Fig. 2 of the Major Inorganic Constituents section. What are the two predominant anions in Lithia water? Using the standard reduction tables in Analytical Chemistry 2.0, is there any reason to expect these anions to react with dissolved oxygen at concentrations expected in Lithia water? Provide appropriate support for your answer.
Q5. Using the standard reduction tables in Analytical Chemistry 2.0, is there any reason to expect main group cations to react with transition metal cations present in Lithia water? Provide appropriate support for your answer.
Q6. Briefly summarize the redox chemistry that according to your analysis can take place among the major inorganic cations in Lithia water.
Solubility Equilibria – First Principles
The solubility rules for inorganic salts in water are provided below:
Rule 1. All compounds of Group IA elements (the alkali metals) are soluble.
Rule 2. All ammonium (NH4+) salts are soluble.
Rule 3. All nitrate (NO3-), chlorate (ClO3-), perchlorate (ClO4-), and acetate (CH3COO- or C2H3O2-) salts are soluble.
Rule 4. All chloride (Cl-), bromide (Br-), and iodide (I-) salts are soluble except for those of Ag+, Pb2+, and Hg22+.
Rule 5. All sulfate ( SO42-) compounds are soluble except those of Ba2+, Sr2+, Ca2+, Pb2+, Hg22+. The Hg2+, Ca2+ and Ag+ sulfates are only moderately soluble.
Rule 6. All hydroxide (OH-) compounds are insoluble except those of Group I-A (alkali metals) and Ba2+, Ca2+, and Sr2+.
Rule 7. All sulfide (S2-) compounds are insoluble except those of Groups I-A and II-A (alkali metals and alkaline earths).
Rule 8. All sulfites (SO32-), carbonates (CO32-), chromates (CrO42-), and phosphates (PO43-) are insoluble except for those of NH4+ and Group I-A (see rules 1 and 2).
Q7. Using the solubility rules provided above and the primary inorganic constituents in Lithia water, which inorganic salts would be most likely to form a precipitate in Lithia water?
The concentration of an ion is controlled in part when a heterogeneous equilibrium is established between ions in solution and a precipitate. It is the establishment of this equilibrium that provides meaning to the concept of solubility. If one starts with pure water and adds an excess of a sparingly soluble solid to the water, the solid will dissolve until the product of the relevant ion concentrations in mol L-1, raised to their stoichiometric coefficients, is equal to the equilibrium constant for the sparingly soluble salt. The equilibrium constant expression for sparingly soluble salts is known as Ksp. An example of a solubility equilibrium is the dissolution of calcium carbonate in water shown in eq 3.
\[\ce{CaCO3 (s) \rightleftharpoons Ca^2+ (aq) + CO3^2- (aq)} \hspace{25px} \mathrm{K_{sp}} = \ce{[Ca^2+][CO3^2- ]} = 4.5 \times 10^{-9}\tag{3}\]
Q8. Why is there no term for the solid calcium carbonate in the Ksp expression?
Precipitation and dissolution processes control the concentration of ionic species, such as those that are being determined in Lithia water.
Solubility Equilibria – Infinite Dilution Conditions
It is important to know how to calculate ion concentrations from solubility equilibria, both under infinite dilution conditions (i.e. zero ionic strength) and under conditions of significant ionic strength. The solubility of compounds in water or other solvents can be expressed in a variety of ways. For our purposes, we will define solubility in the following way:
Solubility is the number of moles of the solid species that dissolve in one liter of solution. This is known as the molar solubility.
The example below involves calculating the solubility of calcium carbonate ignoring the effects of solution pH. Assuming that the calcium carbonate is the only source of ions in solution, it is typically assumed that the ionic strength is negligible, and that the thermodynamically-based solubility product adequately represents the extent to which the dissolution of CaCO3 occurs. The solubility of CaCO3 may be calculated using the following balanced reaction and equilibrium constant expression:
\[\begin{array}{llclcl}
\ce{ &CaCO3 (s) &⇌ &Ca^2+ (aq) &+ &CO3^2- (aq)}\\
\ce{Initial &--- & &0 & &0}\\
\ce{Change &-X & &+X & &+X}\\
\ce{Equilibrium &--- & &X & &X}
\end{array}\nonumber\]
\[\mathrm{K_{sp}=4.5\times10^{-9}=[Ca^{2+}][CO_3^{2-}]=X^2}\nonumber\]
\[\mathrm{X=[Ca^{2+}]=[CO_3^{2-}]=\sqrt{4.5\times10^{-9}}=6.7\times10^{-5}\:M}\nonumber\]
Note, in this case, for each mole of solid calcium carbonate that dissolves, one mole of calcium ion and one mole of carbonate ion is found in solution. Because the relationship is 1:1, the concentration of calcium ion and concentration of carbonate ion is each equal to the solubility of calcium carbonate. If the salt has a stoichiometry that is not 1:1 (e.g., Ca3(PO4)2), the concentration of the calcium ion and concentration of the phosphate ion are not directly equal to the solubility of the solid in water.
Q9. Write expressions that relate the concentration of calcium ion and the concentration of phosphate ion to the solubility of calcium phosphate in water.
These equilibrium calculations can also be applied to all sparingly soluble salts under different experimental conditions. For example, pH has a pronounced effect on the solubility of metal hydroxides. The solubility product constant (Ksp) is tabulated in Table 1 for a series of insoluble and slightly soluble metal hydroxides. The solubility products for the corresponding carbonate salts are also included in Table 1.
Table 1. Solubility product constants for insoluble hydroxide& carbonate salts that could control the solubility of metal ions measured in Lithia water.
|
Metal Cation |
Solubility Equilibrium |
Ksp |
|---|---|---|
|
Al3+ |
\(\ce{Al(OH)3 (s) ⇌ Al^3+ (aq) + 3 OH- (aq)}\) |
4.6 x 10-33 |
|
No pure aluminum carbonate exists |
||
|
Ca2+ |
\(\ce{Ca(OH)2 (s) ⇌ Ca^2+ (aq) + 2 OH- (aq)}\) |
6.5 x 10-6 |
| \(\ce{CaCO3 (s) ⇌ Ca^2+ (aq) + CO3^2- (aq)}\) |
4.5 x 10-9 |
|
|
Fe2+ |
\(\ce{Fe(OH)2 (s) ⇌ Fe^2+ (aq) + 2 OH- (aq)}\) |
8 x 10-16 |
| \(\ce{FeCO3 (s) ⇌ Fe^2+ (aq) + CO3^2- (aq)}\) |
2.1 x 10-11 |
|
|
Fe3+ |
\(\ce{Fe(OH)3 (s) ⇌ Fe^3+ (aq) + 3 OH- (aq)}\) |
1.6 x 10-39 |
|
No stable ferric carbonate exists |
||
|
Mg2+ |
\(\ce{Mg(OH)2 (s) ⇌ Mg^2+ (aq) + 2 OH- (aq)}\) |
7.1 x 10-12 |
| \(\ce{MgCO3 (s) ⇌ Mg^2+ (aq) + CO3^2- (aq)}\) |
3.5 x 10-8 |
Q10. Calculate the solubility of calcium hydroxide in a solution that has a pH of 12. Note: The pH has been adjusted to 12 by some other means than the dissolution of calcium hydroxide. If calcium hydroxide is added to water at pH of 12, some of the calcium hydroxide will dissolve to satisfy the Ksp equilibrium expression.
Q11. What is the molar solubility and mass solubility (i.e. solubility in grams of solid per liter of solution) of each of the metal hydroxides listed in Table 1 at pH values of 10.0, 6.0, and 2.0? How is the solubility of a metal hydroxide affected by pH? Offer an explanation for this behavior using LeChatelier’s principle.
Q12. Integrating the ideas of redox chemistry and solubility expressed in this module, summarize the chemical and physical changes you would expect over time in a sample of Lithia water? What sample preparation step could be used to prevent such changes from occurring?
Q13. Collection of samples for trace metal analysis in drinking water is described in the Sample Collection, Preservation and Storage section of EPA method 200.5, Determination of Trace Elements in Drinking Water by Axially Viewed Inductively Coupled Plasma - Atomic Emission Spectrometry (http://www.epa.gov/nerlcwww/ordmeth.htm). After reading this document, suggest a general approach to preserving Lithia water prior to analysis. Would this work for all analytes of interest in Lithia water? Explain.
Solubility Equilibria – Advanced Topics
Based on the simple solubility equilibrium calculation for calcium carbonate in the Solubility Equilibria – First Principles section, the concentration of calcium ion in Lithia water would be 67 μM, or 2.7 mg L-1. However, there are three problems with this approach:
- According to the carbonic acid ladder diagram, the predominant species is not the carbonate ion but the bicarbonate ion at the pH that is normally measured for Lithia water.
- The ionic strength of Lithia water is not zero.
- Lithia water contains dissolved CO2 (i.e. Lithia water is naturally carbonated), which reacts with water to form carbonic acid. According to LeChatelier’s principle, the dissociation of protons from carbonic acid shifts the carbonate equilbrium in Lithia water favoring the protonation of carbonate ion to form bicarbonate ion.
Problem 1 - Alpha Values
n titratable protons, there are (n+1) alpha values as shown in eq 4:
\[1= α_{H_n A}+ α_{H_{(n-1) A^-}} +α_{H_{(n-2) A^{-2}}} +...+α_{A^{-n}}\tag{4}\]
In eq 4, \(α_{H_n A}\) is the fraction of the analytical concentration for the undissociated acid (no protons lost), \(α_{H_{(n-1) A^-}}\) is the fraction of the analytical concentration for the weak acid that has lost one proton, \(α_{H_{(n-2) A^{-2}}}\) is the fraction of the analytical concentration for the weak acid that has lost two protons, and \(α_{A^{-n}}\) is the conjugate weak base with no remaining protons.
Equations to calculate alpha values as a function of [H+] and Ka are provided without proof below:
Monoprotic Acid
\[\mathrm{\alpha_{HA} = \dfrac{[H^+]}{[H^+] + K_a }}\nonumber\]
\[\mathrm{\alpha_{A^-} = \dfrac{K_a }{[H^+] + K_a }}\nonumber\]
Diprotic Acid
\[\mathrm{\alpha_{H_2A} = \dfrac{[H^+]^2}{[H^+]^2 + [H^+] K_{a1} + K_{a1}K_{a2}}}\nonumber\]
\[\mathrm{\alpha_{HA^-} = \dfrac{[H^+]K_{a1} }{[H^+]^2 + [H^+] K_{a1} + K_{a1}K_{a2}}}\nonumber\]
\[\mathrm{\alpha_{A^{2-}} = \dfrac{K_{a1}K_{a2}}{[H^+]^2 + [H^+] K_{a1} + K_{a1}K_{a2}}}\nonumber\]
Triprotic Acid
\[\mathrm{\alpha_{H_3A} = \dfrac{[H^+]^3}{[H^+]^3 + [H^+]^2 K_{a1} + [H^+]K_{a1}K_{a2} + K_{a1}K_{a2}K_{a3}}}\nonumber\]
\[\mathrm{\alpha_{H_2A^-} = \dfrac{[H^+]^2K_{a1}}{[H^+]^3 + [H^+]^2 K_{a1} + [H^+]K_{a1}K_{a2} + K_{a1}K_{a2}K_{a3}}}\nonumber\]
\[\mathrm{\alpha_{HA^{2-}} = \dfrac{[H^+]K_{a1}K_{a2}}{[H^+]^3 + [H^+]^2 K_{a1} + [H^+]K_{a1}K_{a2} + K_{a1}K_{a2}K_{a3}}}\nonumber\]
\[\mathrm{\alpha_{A^{3-}} = \dfrac{K_{a1}K_{a2}K_{a3}}{[H^+]^3 + [H^+]^2 K_{a1} + [H^+]K_{a1}K_{a2} + K_{a1}K_{a2}K_{a3}}}\nonumber\]
The value of this approach is that if one knows the pH and the weak acid dissociation constants for a particular weak acid, the alpha values for all forms of the weak acid can be calculated.
Q14. Calculate the alpha values for the three carbonic acid species (H2CO3, HCO3-, CO32-) at pH 6.4. Do these values agree with your predictions based on the carbonic acid ladder diagram?
The alpha values you calculated in Q14 provide the relative concentration of the various species in the carbonic acid equilibrium. A useful application of these alpha values is in the calculation of the calcium ion concentration using the calcium carbonate solubility equilibrium expression. In the previous section, the bicarbonate concentration was determined to be 0.06736 M, based on the analysis engraved on the plaque in the Major Inorganic Constituents section.
Q15. Given that the pH of the Lithia water is 6.4, what is the total concentration of all species in the carbonic acid equilibrium?
Q16. What is the concentration of carbonate ion?
Q17. Using this concentration of carbonate, calculate the concentration of calcium that you would expect in Lithia water from the dissolution of calcium carbonate.
Q18. Compare this value to the value of calcium on the plaque.
This concentration is approximately fifteen times lower than the calcium ion content determined, which indicates that the acid-base chemistry of the carbonic acid system is not the only factor that controls the calcium ion concentration in Lithia water.
Problem 2 - Effect of Ionic Strength on Solubility
The extent to which a precipitate dissociates into solution phase ions is also affected by the ionic strength of the solution. The equilibrium constants compiled in the back of Analytical Chemistry 2.0 are based on an ionic strength of zero (i.e. infinite dilution conditions). If the ionic strength of a solution is greater than zero, there is a measurable concentration of supporting electrolyte in the solution. Ionic strength is defined in the equation below:
\[\mathrm{\mu=\sum C_iz_i^2}\nonumber\]
In the ionic strength equation, µ represents the ionic strength of the solution (mol L-1) , Ci represents the concentration (mol L-1)of each ionic species i and zi represents the charge of each ionic species i.
Q19. What effect would raising the ionic strength of a solution have on the solubility of a sparingly soluble salt? Think about what relatively high concentrations of other ions (e.g., Na+ and Cl-) might have in a solution containing smaller amounts of Ca2+ and CO32-.
In the case of the solubility of a sparingly soluble salt, the analyte ions not only interact with the solvent via ion-dipole interactions, but they also experience coulombic interactions with the supporting electrolyte. The ions from the supporting electrolyte partially screen the charge of each analyte ion, which is shown in Chapter 6, Section 9 of Analytical Chemistry 2.0. These interactions decrease the “effective concentration” or activity of the analyte ions. The relationship between the analytical concentration and the effective concentration (activity) is shown below:
\[a_x=γ_x [X]\nonumber\]
In the equation above, ax represents the activity of an analyte ion in mol L-1, [X] is the analytical concentration in mol L-1, and γx is the activity coefficient. As the ionic strength of a solution increases, the activity coefficient decreases, and so does the activity of an analyte.
The relationship between activity and concentration is important because chemical equilibria are based on analyte activity and not analyte concentration. Therefore, the equilibrium constant expression for the general reaction:
\[\mathrm{aA (aq) + bB (aq) \rightleftharpoons cC (aq) + dD (aq)}\nonumber\]
is:
\[\mathrm{K_{eq}=\dfrac{a_C^c a_D^d}{a_A^a a_B^b}=\dfrac{γ_C^c γ_D^d}{γ_A^a γ_B^b}\dfrac{[C]^c [D]^d}{[A]^a [B]^b}}\nonumber\]
The value Keq is called the thermodynamic equilibrium constant because the constant is based on activities, which are only equal to concentrations under infinite dilution conditions (µ = 0). Many analytical methods determine concentrations, not activities, and the ionic strength of the solutions analyzed tends to be significantly greater than zero. Rearranging the equation above results in the following equilibrium constant expression:
\[\mathrm{{K}'_{eq}=K_{eq} \dfrac{γ_A^a γ_B^b}{γ_C^c γ_D^d}=\dfrac{[C]^c [D]^d}{[A]^a [B]^b }}\nonumber\]
In the equation above, K’eq is called the concentration-based equilibrium constant. This equilibrium constant describes the favorability of a reaction in a system with an ionic strength greater than zero.
To calculate the concentration-based equilibrium constant, the activity coefficient for each analyte ion is needed. A common approach for the estimation of activity coefficients is the Debye-Hückel equation:
\[\mathrm{\log γ_x= \dfrac{-0.51 z^2 \sqrt μ}{1+3.3α_x \sqrt μ}}\nonumber\]
In the Debye-Hückel equation, z is the charge on the analyte ion, αx is the hydrated ion diameter of the analyte in nanometers, and µ is the ionic strength in mol L-1. Once the activity coefficient for each analyte ion is calculated, the concentration-based equilibrium constant may be calculated and used to solve chemical equilibrium problems.
Most measurements made in the analytical chemistry laboratory detect the concentration of the analyte. A spectroscopic measurement based on the Beer-Lambert law is an example of the direct proportionality between analyte concentration and absorbance. Interestingly, ion selective electrodes (ISEs) detect the activity of an analyte and not its concentration. Although the effect of ionic strength on the activity coefficient, and therefore on the analyte activity, is expressed through the Debye-Hückel equation, it is common practice to add a high ionic strength solution to the calibration standards and samples so that the ionic strength is constant for all the measurements. Under these conditions, the activity coefficient for each solution will be the same, and the calibration plot can be used to determine the analyte concentration.
Q20. Calculate the molar solubility of AgCl under infinite dilution conditions (i.e. µ = 0) with its solubility in 0.10 M NaNO3. The hydrated ion diameters and thermodynamic equilibrium constants may be obtained from Analytical Chemistry 2.0.
Q21. Calculate the concentration-based equilibrium constant for CaCO3, H2CO3, and HCO3-, and use these constants to predict the calcium concentration in Lithia water at pH 6.4. The ionic strength of Lithia water may be calculated from the solute molarities reported on the plaque from the Major Inorganic Constituents section, and the hydrated ion diameters and thermodynamic equilibrium constants may be obtained from Analytical Chemistry 2.0.
Problem 3 – Effect of dissolved CO2
Air contains carbon dioxide gas that dissolves to some extent in water. The Lithia spring water also contains some dissolved carbon dioxide that comes from carbon dioxide gas entrained in the rocks that feed the springs. When carbon dioxide dissolves in water, a chemical reaction occurs that results in the formation of a weak acid, carbonic acid.
\[\ce{CO2 (aq) + H2O (l) ⇌ H2CO3 (aq)}\nonumber\]
Q22. Remembering that the Lithia water contains carbonate ion, use LeChatelier’s principle to explain what effect the addition of carbonic acid has on the concentration of carbonate ion in Lithia water?
Q23. Considering your answer to the preceding question, what effect would the addition of carbonic acid have on the solubility of calcium carbonate and other sparingly soluble carbonate salts?
Q24. The concentration of calcium ion based on the 1915 analysis of Lithia water is 347 mg L-1. Assuming that the calcium ion concentration in Lithia water is controlled by the carbonate ion concentration in Lithia water, calculate the carbonate ion concentration in Lithia water using the concentration-based equilibrium constant for calcium carbonate.


