Analysis of Anions by Spectrophotometry
- Page ID
- 219078
Purpose: The purpose of this assignment is to introduce fundamental concepts of visible (VIS) spectrophotometry as it pertains to the measurement of analytes in water samples. Phosphate will be the specific example used.
Learning Outcomes:
At the end of this assignment, students will be able to:
- Select the appropriate wavelength to collect spectra.
- Evaluate the selectivity and detection limits of the method.
- Use UV-VIS spectrophotometry as a quantitative tool (use Beer’s law to calibrate the spectrophotometer and determine the concentration of an unknown amount of phosphate in a water sample).
For a discussion of fundamental concepts of UV-VIS spectrophotometry check the following links:
- Analytical Chemistry 2.0 – Chapter 10
- Understanding Chemistry – UV-VIS Spectroscopy
Often, to assess water quality, anions such as nitrates and phosphates need to be quantitatively determined at concentrations in the parts per million (ppm) range or lower. One way to measure low concentrations of many species is to use a spectrophotometric analysis. A spectrophotometric analysis is performed by illuminating the sample with radiation in the visible or ultraviolet range and detecting the amount of light that reaches the detector. Depending on the chemical properties of the species in the sample, some light will be absorbed while the remainder will be transmitted to the detector where it is converted to an electrical signal that is proportional to the amount of light absorbed, called the absorbance. By constructing a standard calibration curve using known concentrations of the analyte under investigation, a relationship can be developed between the absorbance of the analyte at a specific wavelength and its concentration. By measuring the absorbance of an unknown sample and comparing it to the calibration plot, the concentration of the analyte in the sample can be determined. This module will address the spectrophotometric analysis of phosphate.
Q1: Draw a block diagram of the components of a spectrophotometer.
When a beam of electromagnetic radiation passes through a sample, most of the radiation is transmitted but, at specific wavelengths, the radiation may be absorbed by chemical constituents within the sample. For an atom or molecular species, the absorption of light causes valence electrons to be excited from lower to higher energy states. For this transition to occur, the energy provided by the radiation has to match the difference in energy (ΔE) between the lower and higher energy states.
Q2: What molecular properties must a compound have in order to absorb UV-VIS radiation?
Q3: Do you think phosphate ions have the ability to absorb UV or visible radiation? Justify your answer.
Many species, including phosphates, can be analyzed by visible spectrophotometry by reacting them with other species, thus forming a new compound that absorbs light at a specific wavelength. The general approach to any spectrophotometric analysis is the same with differences in reagents and chemical reactions used to generate the light absorbing species.
Exploring fundamental relationships in spectrophotometric methods
When light traveling through a sample is absorbed, the radiant power of the beam decreases from P0 to P, where P0 represents the radiant power of the beam before the sample and P the power after the sample. The fraction of incident radiation transmitted through the solution is called transmittance and is represented by the equation:
\[\mathrm{T = P/P_0}\nonumber\]
The absorbance of a solution is related to the transmittance through a logarithmic relationship:
\[\mathrm{A = - \log T = - \log P/P_0 = \log P_0/P}\nonumber\]
Before moving on to the analysis of phosphate, we will examine some dye solutions to explore fundamental relationships within spectrophotometric methods.
For the following activity you can either use a real spectrophotometer or the simulator at the following website http://web.mst.edu/~gbert/ColorScan/Spectrophotometry.HTML
Prepare solutions of red dye and blue dye and record their spectra between 400 and 700 nm.
- At which wavelength does the blue dye absorb the most? This wavelength is referred to as λmax
- At which wavelength does the red dye absorb the most?
- What is the value of the transmittance at λmax for each dye? What is the value of the absorbance?
- What wavelength would you choose to quantitatively determine the concentration of the red dye? Why did you choose this wavelength?
- What wavelength would you choose to quantitatively determine the concentration of the blue dye? Why did you choose this wavelength?
Determining method’s selectivity
A method’s selectivity refers to the extent to which it can determine particular analyte(s) in a complex mixture without interference from other components in the mixture (4,5).
Q4: Is it possible to determine the concentration of the blue dye if it is contaminated with some of the red dye?
Q5: When does it become possible to selectively measure the blue dye in presence of the red dye?
Relationship between absorbance and concentration
At the heart of a quantitative estimation of phosphates using UV-VIS spectrophotometry is Beer’s law which states the linear relationship between absorbance and concentration according to the equation:
\[\mathrm{A = εbc}\nonumber\]
where
A is the absorbance
ε is the molar absorptivity - a measure of the probability of the transition – the molar absorptivity is largest at λ max, meaning that this is the most probable transition
b is the path length of the cuvette
c is the concentration
Q6: Draw a representative plot of A versus c if the spectrophotometer is set to λmax.
Q7: How could you use this plot to determine the concentration of an unknown?
Analyzing phosphate by visible spectrophotometry
Now that we have developed general background information on spectrophotometric measurements, we can explore specific features of its use in the analysis of phosphate ion.
In question 3 you were asked if phosphate has the ability to absorb UV or VIS light. Most likely the anion does absorb light but such absorbance is not sensitive and specific enough to base a quantitative method of analysis. One strategy to improve analysis sensitivity is to react the species, in this case phosphate, with a reagent that forms a new molecule capable of absorbing light at a specific wavelength. The amount of phosphorous as orthophosphate (PO43-) present in a water sample may be best determined by the ascorbic acid/molybdenum blue method (1-3). In this method, ammonium paramolybdate, (NH4)6Mo7O24∙4H2O, reacts with orthophosphate in acidic solution and in the presence of potassium antimonyl tartrate, C8H4K2O12Sb2, to produce phosphomolybdic acid, H3[P(Mo3O10)4]. This acid, in turn, is reduced by ascorbic acid, C6H8O6, to produce intensely colored molybdenum blue. “Molybdenum blue” is actually not a single compound, but a complex mixture of polyoxomolybdate (POM) ions. The role of potassium antimonyl tartrate is as a catalyst.
Below is a spectrum recorded on phosphate solutions of increasing concentrations after reaction with the molybdenum blue reagent.
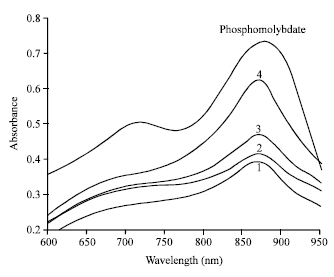
Q8: What wavelength would you choose to quantitatively determine orthophosphate by the molybdenum blue method?
Assessment of possible interferences
Q9: What potential interferences would limit the measurement of orthophosphate using the molybdenum blue method?
Q10: What steps could you take in the sample preparation to decrease the chances of interfering species in the orthophosphate analysis?
Q11: Looking at the interferences listed in EPA method 365.1 will any of these affect the way you will analyze your sample for orthophosphate?
Preparing calibration standards for phosphate analysis
You are now going to prepare a set of phosphate calibration standards and use them to calibrate the spectrophotometer.
Q12: Given that a stock solution of 3 mg/L phosphate (PO43-) is equivalent to a concentration of 1 mg/L of elemental phosphorous (P), how many milliliters will you have to pipette to prepare 25.00 mL of the following six standards?
|
Standard concentration (mg P/L) |
Milliliters of phosphate standard to pipette |
|---|---|
|
0.00 |
|
|
0.04 |
|
|
0.08 |
|
|
0.12 |
|
|
0.15 |
|
|
0.20 |
Q13: What is the purpose of preparing the standard at 0.00 mg/L P? How is it used in the analysis?
Q14: The second standard is 0.04 mg P/L. How many parts per million (ppm) of P does this correspond to?
The following absorbance values were recorded when analyzing the six standards prepared above:
|
Standard concentration (mg/LP) |
Absorbance |
|---|---|
|
0.00 |
0.000 |
|
0.04 |
0.021 |
|
0.08 |
0.039 |
|
0.12 |
0.052 |
|
0.15 |
0.078 |
|
0.20 |
0.102 |
Use Excel or equivalent software to plot the standard calibration curve.
Q15: What relationship do you observe between absorbance and concentration?
Q16: What parameter allows us to determine whether there is a good fit between absorbance and phosphate concentration?
Once the calibration is completed, the next step is to analyze unknown water samples for phosphate concentration. Suppose that three replicate water samples from one of the ponds at End Creek are analyzed and their absorbance is 0.025, 0.027 and 0.023, respectively.
Q17: What is the average concentration of phosphate (expressed in mg P/L) in the pond water?
Q18: What parameter allows you to assess the reproducibility of the measurement?
Evaluating the detection limit of the method
The detection limit is the smallest concentration or absolute amount of analyte which, with a stated probability, can be distinguished from a suitable blank value (5). If we define Smb as the average signal for a method blank, and σmb as the method blank’s standard deviation, the detection limit (S)DL is calculated as follow:
\[\mathrm{(S)_{DL} = S_{mb} + 3σ_{mb}}\nonumber\]
Q19: How would you determine the average signal and the associated standard deviation of the method blank using the UV-VIS spectrophotometer?
Q20: If you determined that the average signal from the method’s blank is 0.0081 and the standard deviation is 0.0006, what is the detection limit of the method?
Q21: Would you be able to detect phosphate in the pond water mentioned above if the absorbance of an unknown sample was 0.003?
Automated methods
Most water labs currently use automated methods of analysis for phosphate, nitrates and other routine analytes. A typical approach is based on flow-injection analysis.
Q22: What are the likely advantages of automating a method?
References
- APHA. 1992. Standard methods for the examination of water and wastewater. 18th ed. American Public Health Association, Washington, DC.
- EPA method 365.1 – Determination of phosphorous by semi-automated colorimetry –revision 2.0, August 1993.
- Volunteer Stream Monitoring: A Methods Manual
http://water.epa.gov/type/rsl/monitoring/stream_index.cfm (access on 02-23-2014) - Vessman, J.; Stefan, R.; Van Staden, J.; Danzer, K.; Lindner, W.; Thorburn Burns, D.; Fajgeli, A.; and Muller, H. 2001. Selectivity in Analytical Chemistry, Pure Appl. Chem., Vol. 73, No. 8, pp. 1381–1386.
- IUPAC Compendium of Chemical Technology, Electronic Version, http://goldbook.iupac.org/D01629.html (access on 02-23-2014)


