Ion Chromatography
- Page ID
- 227146
This instructor’s guide includes sample answers to the questions in the IC module, and additional information at times.
I have given this module as a take home assignment for the students to work on and read through, then had them present their answers to each other in small groups. This resulted in the students having discussion on where their answers were different and did well in getting to the underlying principles.
Q1. Write the chemical reaction for the association of A- and B- with the ion exchange resin.
\[\ce{Resin^+-E- + A- <=> Resin^+-A- + E- }\nonumber\]
\[\ce{Resin^+-E- + B- <=> Resin^+-B- + E- }\nonumber\]
Q2. Write the chemical reaction for the elution of A- and B- from the ion exchange resin.
\[\ce{Resin^+-A- + E- <=> Resin^+-E- + A- }\nonumber\]
\[\ce{Resin^+-B- + E- <=> Resin^+-E- + B- }\nonumber\]
Q3. Write the equilibrium expression constant for A- and B-. In chromatographic separations, this term (Kc) is referred to as the distribution coefficient.
Q4. Do you think the magnitude of the distribution coefficients are the same for A- and B-? Why or why not?
If they are different ions, then they would have different distribution constants, because they would have different affinities for the resins. These differences will arise due to differences in size, charge, and other characteristics.
Q5. If the distribution coefficient of A-( ) is smaller than the distribution coefficient of B-(
) is smaller than the distribution coefficient of B-( ), draw a sketch of the elution process that is similar to the figure in Step 1 but at a point where A- and B- are partway through the column.
), draw a sketch of the elution process that is similar to the figure in Step 1 but at a point where A- and B- are partway through the column.
Q6. Suppose B- had a very strong affinity for the resin, what would happen to its elution time?
As the affinity for the resin increases the elution time increases. Thus you could get to a point where the elution time is so long it does not appear to come off the column.
As the sample is injected onto the column, the two different analytes briefly displace the eluent as the counter-ion to the charged resin. The analyte is briefly retained at the fixed charge on the resin surface. The analytes are subsequently displaced by the eluent ions as the eluent is added to the column. The different affinities (see the chemical reactions in the basic process section) are the basis for the separation. The Kf value of each reaction is also known as the selectivity coefficient. The greater the difference between the Kf values for the two analytes, the more the two analytes will be separated during the ion chromatography process. In reality, the interaction between the solvent and the analyte can also have an impact on the order each analyte is eluted. For a more in-depth analysis of predicting the retention order see the material by Dr. Thomas Wenzel. (http://www.bates.edu/x65385.xml)
Q7. Is this a desirable or undesirable situation if you were trying to analyze A- and B- in a mixture?
You want have a difference in elution times, but you do not want the affinity to be so strong that the ion does not readily come off the column. The longer the elution time the more peak broadening will happen (this links to later brief information on peak broadening.)
Q8. Is there a situation you can think of when it might be desirable for B- to have a very strong affinity for the resin?
Water filtration systems, such as water softeners, usually have an ion exchange resin where the affinities are strong enough to effectively remove ions from the water. The Mg2+ or Ca2+ displaces the Na+ on the resin. The affinity for the Mg2+ or Ca2+ ions needs to be much stronger than the affinity for Na+ in order to effectively soften the water. In sample pretreatment you may want very strong affinities to bind all of the ions and then extract with a different solvent.
Q9. If you want to separate cations, what would be different about the stationary and mobile phases?
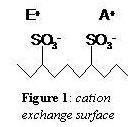
The resin would need to be negatively charged, and the mobile phase would be positively charged. The common cation exchange resins are based on either polystyrene-divinylbenzene (PS-DVB) or methacrylate polymers. The surface of these polymers (Figure 1) is functionalized with a negatively charged sulfonated group (-SO3- ). The cation in the eluent or the analyte of interest is the counter-ion in the vicinity of the charged functional group.
The surface of the polymer is functionalized with a quaternary amine (-N+R3) for anion exchange (see Figure 2). The quaternary amine provides a positive charge to the surface, attracting negatively charged anions in the liquid phase. Just like the cation exchange resin, the anion of the eluent or the analyte of interest exists as the counter-ion in the vicinity of the positive charge residing on the amine.
Q10. Would water flow easily through a column containing very fine particles?
As the resin particle become finer, they pack together closer, leaving less room for the solution to flow through. This, in conjunction with the more resin-solvent interactions, will increase the resistance to flow. You then have to apply pressure to force the solutions through the column.
Q11. If not, how could you get the water through the column?
As the water becomes less likely to flow, you need to apply a force to push the water through the column. This is done with a pump that can handle higher pressures (often 200-1000 psi) such as a double piston high pressure pump to force the mobile phase through the column.
Q12. Considering that the column is packed with very fine particles, what must be done to surface water samples before injecting them onto the column?
If the column particles are very fine, the surface water sample will likely need filtered to remove any detritus that would foul up the column. Surface water samples are filtered through at least 0.45 µm filters but may be filtered through as small as a 0.20 µm, similar to how other solutions are filtered for use in IC. The filter type is one that must not introduce an error.
Q13. Can you think of a way to detect the presence of ionic substances in water?
The most common factor to all ionic substances is that they have a charge, thus a detection method that is based on measuring the charge is frequently used. Ions in solution conduct electricity, so the conductivity of a solution will change as the concentration of ions change. This is the common basic detection. There are other possibilities that are more selective.
Conductivity would not distinguish between the two ions. Therefore you need the two ions to be separated on the column.
The selectivity in the overall method comes from a good separation of the ions, so the detection method does not need to be very selective. The detection does need to be quantifiable and applicable to a broad range of ions.
Q16. Will the eluent ion respond to the detection method you thought of above to measure the presence of Na+ and Ca2+.
Yes it will. Thus eluent suppression will usually be used.
Q17. How will the chromatogram change as you increase the concentration of A- and B- injected into the column? Make sure to label the axes of your chromatogram.
x-axis is elution time, y-axis is conductivity. As you increase the concentration of an ion, the height and area of the peak corresponding to that ion will increase.
Q18. Since neither axis in the chromatogram you drew above is concentration, how can we calibrate the detector response to determine the concentration of A- and B-?
You have to calibrate the detector by analyzing standard samples with known concentrations of both A- and B-. The conductivity is plotted as a function of concentration to determine the response of the detector.


