3.7: Other Luminescent Methods
- Page ID
- 111614
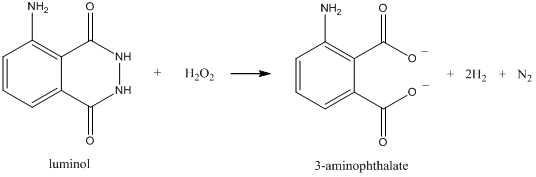
Two other important forms of luminescence are chemiluminescence and bioluminescence. Chemiluminescence refers to a process in which a chemical reaction forms a product molecule that is in an excited state. The excited state product then emits radiation. The classic example of a chemiluminescent process involves the reaction of luminol with hydrogen peroxide (H2O2) in the presence of a catalyst as shown below. The reaction generates 3-aminophthalate in an excited state and it emits a bluish light. The luminal reaction is used in forensics to detect the presence of blood. In this case, the iron from the hemoglobin serves as the catalyst.
Another important example of a chemiluminescent reaction involves the reaction of nitric oxide (NO) with ozone (O3) to produce excited state nitrogen dioxide (NO2*) and oxygen gas. Nitric oxide is an important compound in atmospheric chemistry and, with the use of an ozone generator, it is possible to use the chemiluminescent reaction as a sensitive way of measuring NO.
\[\mathrm{NO = O_3 = NO_2^* + O_2} \nonumber \]
\[\mathrm{NO_2^* = NO_2 + h\nu} \nonumber \]
An important feature of both chemiluminescent reactions above is that peroxide and ozone, which are strong oxidants, have an unstable or energetic chemical bond. Chemiluminescence is a rare process only occurring in a limited number of chemical reactions.
Bioluminescence refers to a situation when living organisms use a chemiluminescent reaction to produce a luminescent emission. The classic example is fireflies. There are also a number of bioluminescent marine organisms.
Triboluminescence is a form of luminescence caused by friction. Breaking or crushing a wintergreen-flavored lifesaver in the dark produces triboluminescence. The friction of the crushing action excites sugar molecules that emit ultraviolet radiation, which is triboluminescence but cannot be seen by our eyes. However, the ultraviolet radiation emitted by the sugar is absorbed by fluorescent methyl salicylate molecules that account for the wintergreen flavor. The methyl salicylate molecules emit the light that can be seen by our eyes.
Finally, light sticks also rely on a fluorescent process. Bending the light stick breaks a vial that leads to the mixing of phenyl oxalate ester and hydrogen peroxide. Two subsequent decomposition reactions occur, the last of which releases energy that excites a fluorescent dye. Emission from the dye accounts for the glow from the light stick.


