24: Photosynthesis
- Page ID
- 150980
Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Photosynthesis Overview
Photosynthesis couples energy from the sun to production of carbohydrates such as glucose, which can be stored and later broken down in glycolysis and the citric acid cycle to repackage energy into ATP molecules.
Photosynthesis takes place on the thylakoids, small structures inside the aqueous interior (stroma) of the chloroplasts. The thylakoid is hollow, filled with an aqueous medium (lumen). The major components of photosynthesis are found on the thylakoid membrane as outlined below:
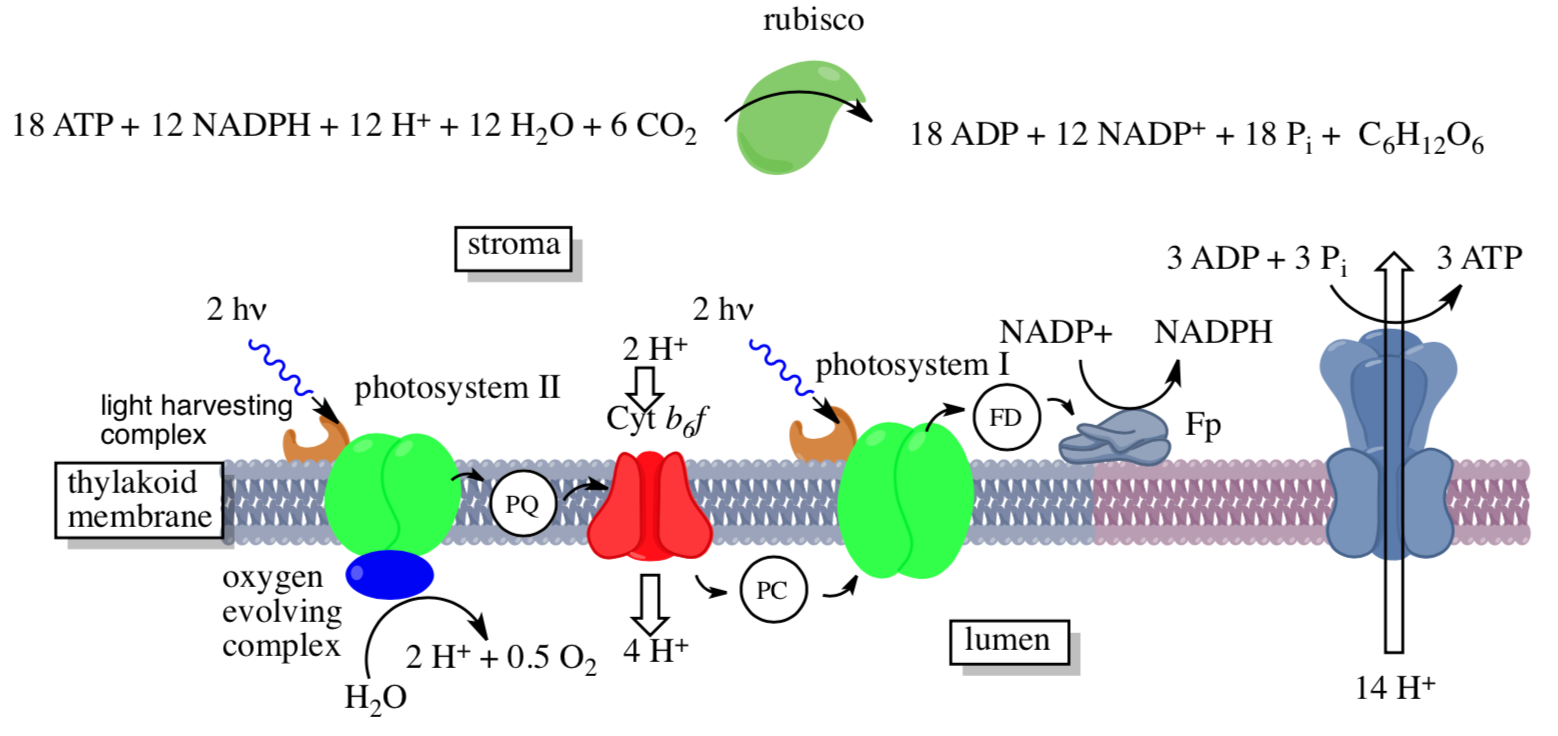
- Use the diagram to match the components to the descriptions:
Photosynthesis Overview: Light Harvesting Complex
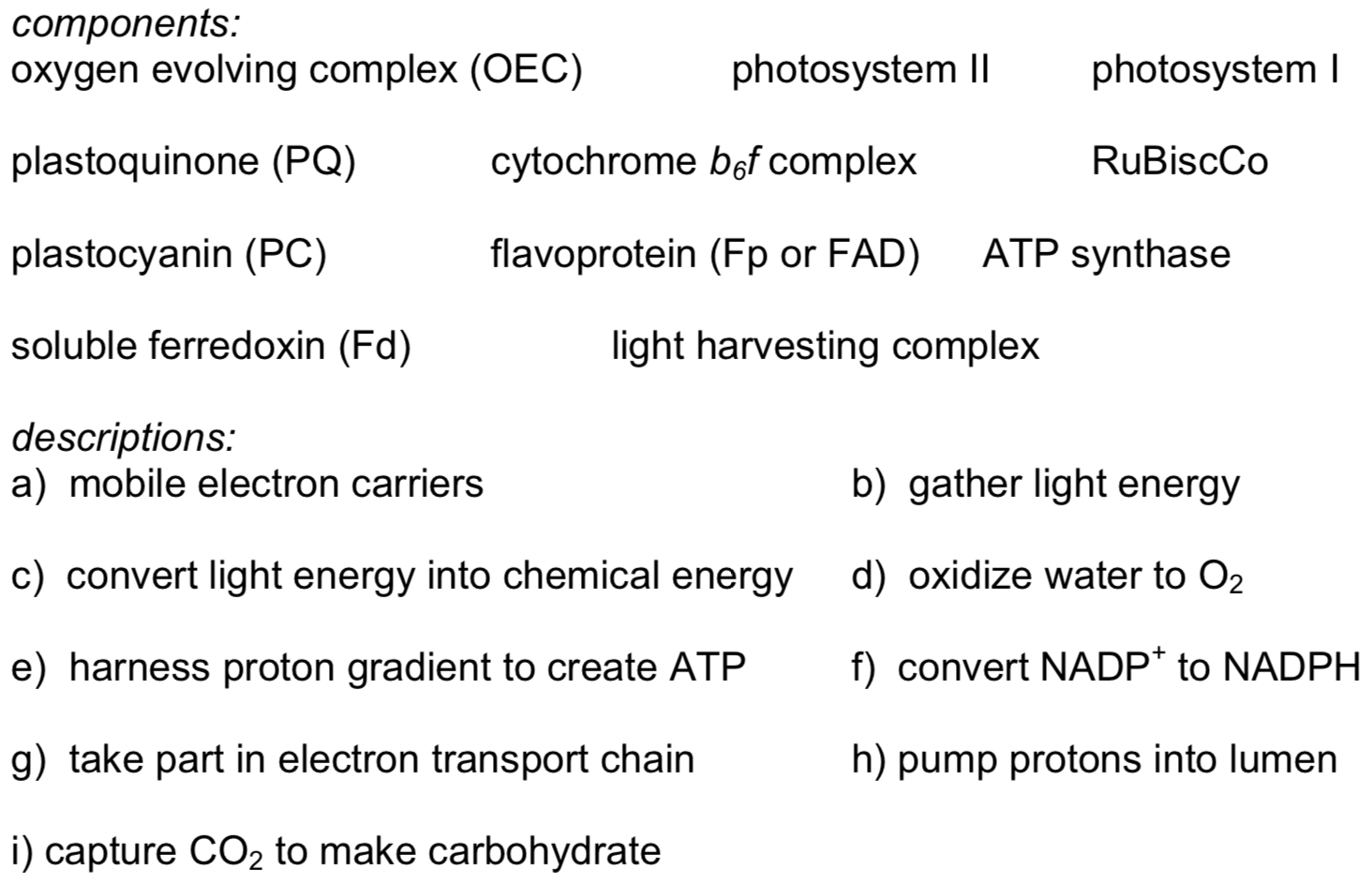
The light harvesting complex is an array of proteins and pigments embedded in the thylakoid membrane. Pigments include carotenoids and chlorophylls, among others.
- What makes these compounds soluble in the membrane?
- What colour(s) would carotene absorb? What colour would it appear?
- What colour(s) would chlorophyll a absorb? What colour would it appear?
- What possible electronic transition is associated with these strong absorption maxima?
- What factor moves their absorption maxima into the visible region?
- Propose a reason for the presence of a variety of pigments in the light harvesting complex.
Photosynthesis Overview: Oxygen Evolving Complex and Electron Chain
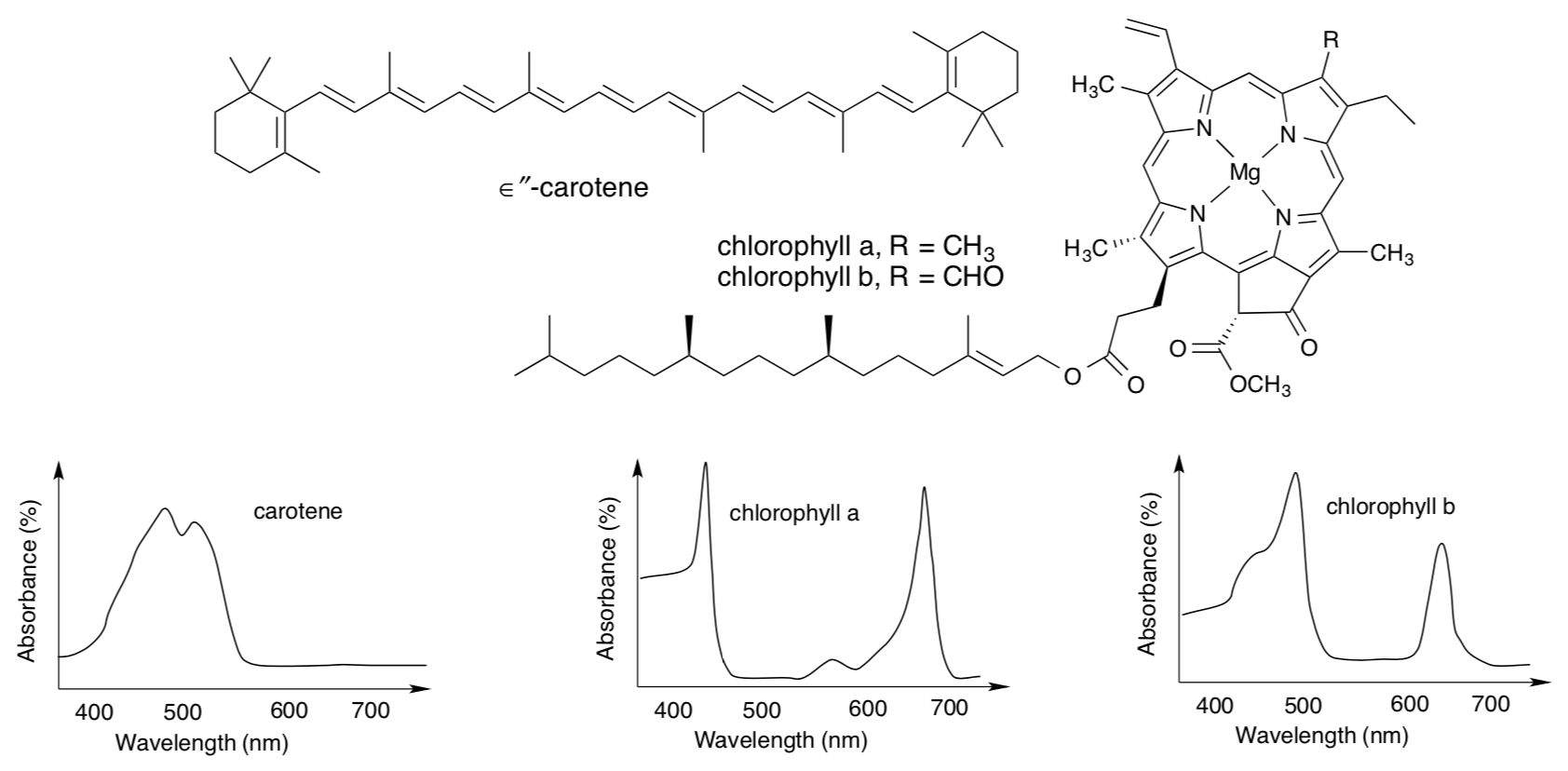
Photosystem II is associated with the following reaction in the oxygen evolving complex (OEC):
- The reaction is the reduction / oxidation of water (circle) and it is very exothermic / exothermic / endothermic / very endothermic (circle)
Absorption of light by photosystem II results in the excitation of an electron. This electron is sent into an electron chain, similar to oxidative phosphorylation. This electron must then be replaced.
- Where does photosystem II get a replacement for its missing electron?
The electron chain is often depicted on a scale of reduction potential, as follows. P680 and P700 are common notations for the chlorophylls in the reaction center.
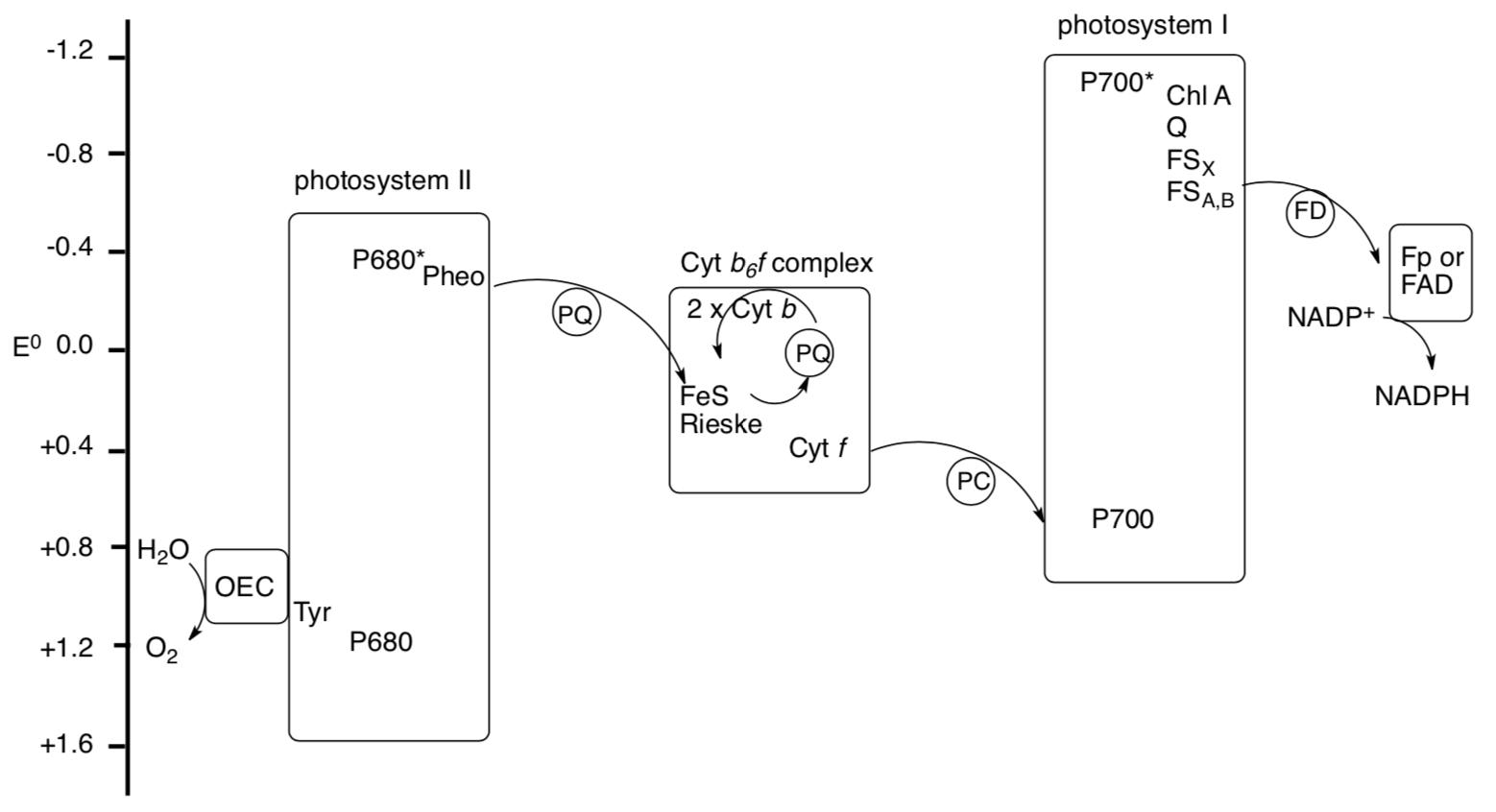
- Trace the electron flow through the picture from water to NADPH. (Note: make sure to include excitation when P680 and P700 absorb photons.)
- Why does reduction potential of the chlorophyll change upon excitation?
- PQ/PQH2 is recycled in the Cytochrome b6f complex, picking up two more protons from the stroma. Suggest roles for the two Cyt b in this process.
Photosynthesis Overview: Carbon Capture and Calvin Cycle
Carbon capture is catalyzed by ribulose bisphosphate carboxylase (RuBisCo) as shown below.
- Fill in missing intermediates and provide mechanisms with curved arrows, with typical biological proton shuttles (e.g. His, Asp...) as needed.
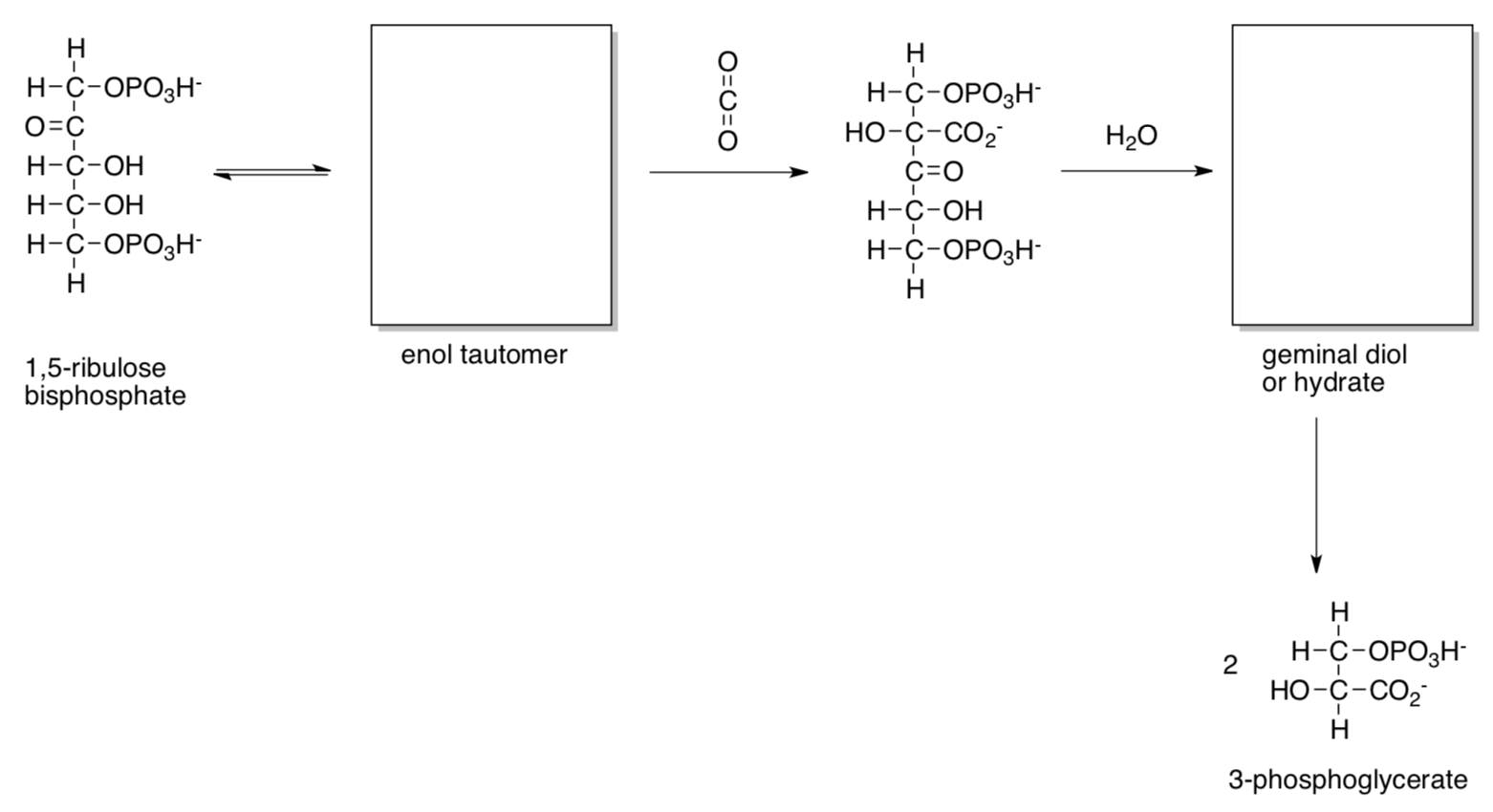
Only a sixth of the 3-phosphoglycerate molecules produced go on to make glucose. The other 5/6 are recycled to make more 1,5-ribulose bisphosphate.
RuBisCo is activated in the presence of ATP and CO2, which results in conformational changes in the protein. Prior to ATP addition, 1,5-ribulose phosphate binds very tightly, inhibiting the enzyme.
- Why might these conditions act as an “on” switch for RuBisCo?
The CO2-based control is linked to a conserved lysine in the protein.
- Show how the lysine might react with a CO2, in the presence of magnesium ion.
- How might this reaction result in conformational change in the protein?
Photosynthesis in Detail
Photosynthesis is one of the most critical reaction pathways in the biosphere
6 CO2(g) + 6H2O(l) -> C6H12O6 (s) + 6O2 (g)
- Which catabolic pathways are involved in the reverse of the above reaction? Where are they localized in the cell?
Photosynthesis involves two pathways, the light and dark reactions.
Light reaction: 6 H2O -> 6 O2 + 12 H+.
- Would you expect this part to be exergonic or endergonic (think about the opposite, burning hydrogen to produce water)?
- What energy source drives the light reaction?
- What other product is missing from the reaction as written?
- Therefore, what type of reaction is this?
Dark reaction (does not need light): 6 CO2(g) + 12 H+ -> C6H12O6 (s).
- Would you expect this part to be exergonic or endergonic (think about the opposite, burning sugar to produce CO2)?
- What common biological energy source drives the dark reaction?
- What other reactant is missing from the reaction as written?
- Therefore, what type of reaction is this?
The overall photosynthetic pathway is shown in the diagram below (reproduced with permission from Kegg Pathways)
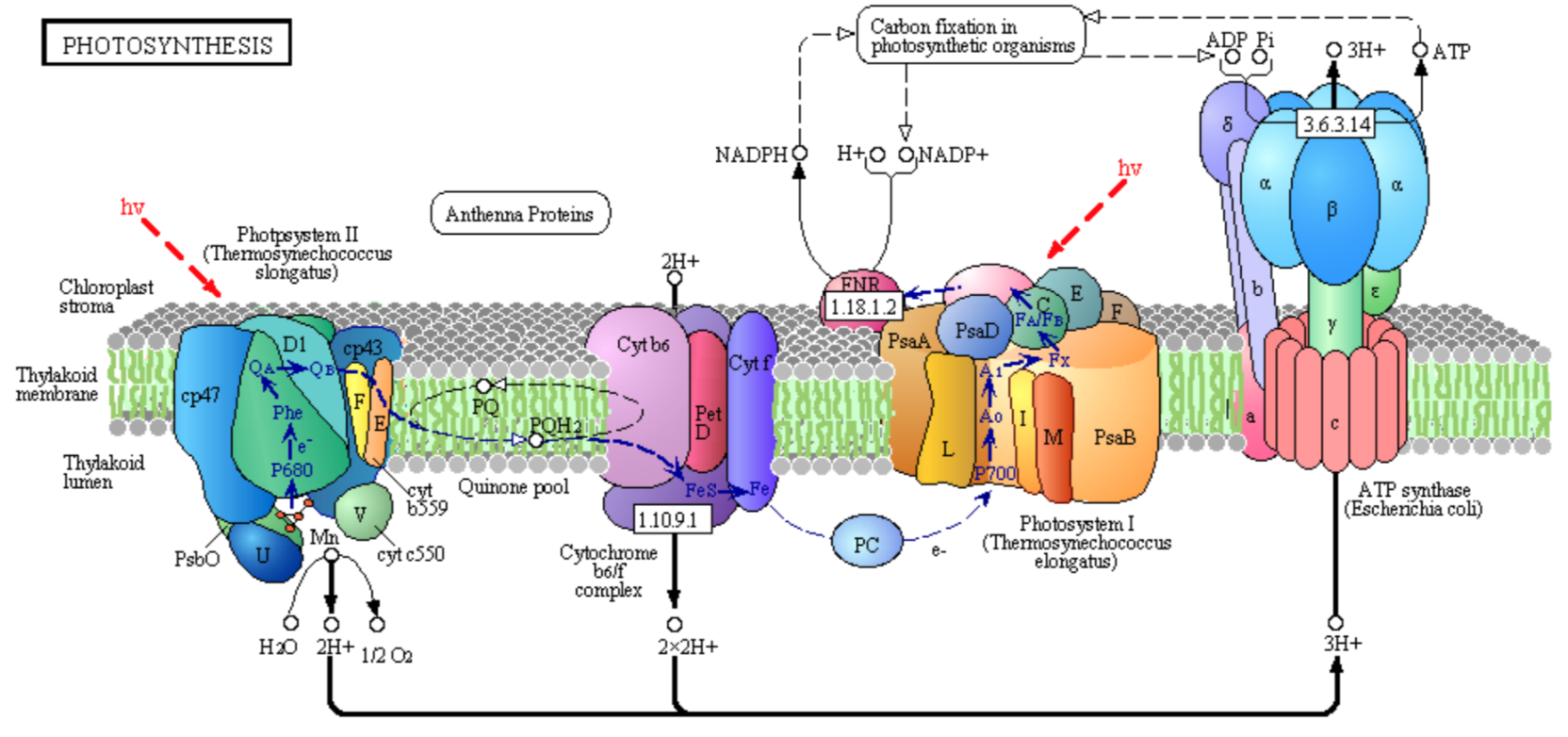
This reaction is catalyzed by enzyme complexes found in the thylakoid membrane of plants and membranes of photosynthetic cyanobacteria (blue-green algae).
- Point out some similarities between the structures and processes used for photosynthesis and those used for oxidative phosphorylation.
- Circle in the figure above all mobile carriers of electrons. Identify them as hydrophobic or hydrophilic.
- What use is made of the NADPH, a phosphorylated version of NADH, that is produced by Photosystem I?
- What use is made of ATP produced by ATP synthase?
- What feature is used to drive ATP synthesis by the ATP synthase?
Before photosynthetic pathways evolved, molecules other than water were oxidized to supply electrons.
- Suggest possible oxidation products for the following reactants:
H2S
Fe2+
Mn2+
- What are the advantages to using H2O as a reactant?
The appearance of photosynthesis on the planet is believed to have produced a major change in the composition of our atmosphere.
- What was the change?
- What other kinds of organisms took advantage of this change?
- What are the advantages to organisms in evolving to use the photosynthetic waste product, O2?
- What might be deleterious consequences of its use?
Photosystem II: The Role of P680
Photosystem II: Chlorophylls
Ref: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2597837/; NIH public access author manuscript,
C. S. Mullins and V. Pecoraro, Coord Chem Rev, 2008, 252(3-4) 416-443
Photosynthetic bacteria and plants have an abundance of molecules that interact with
visible light. The main one found in PSII is chlorophyll a, shown below in X-ray data.

- What main structural feature makes chlorophyll absorb visible light?
There are 35 chlorophylls in PSII. Most are coordinated by an additional His (as shown in the example above), although 7 are coordinated by a water instead.
- Why might there be so many chlorophylls in PSII?
- Why might it be advantageous to have different ligands on different chlorophylls?
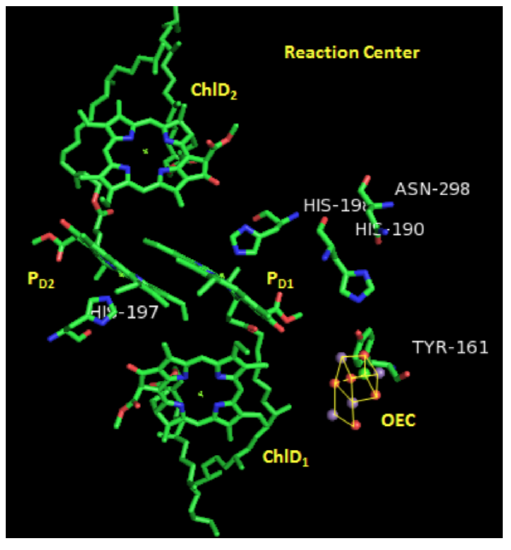
Photosystem II: Reaction Center
Two pairs of chlorophylls, ChlD2 and ChlD2, along with PD1 and PD2, are found near the OEC. These 4 chlorophylls are called the reaction center.
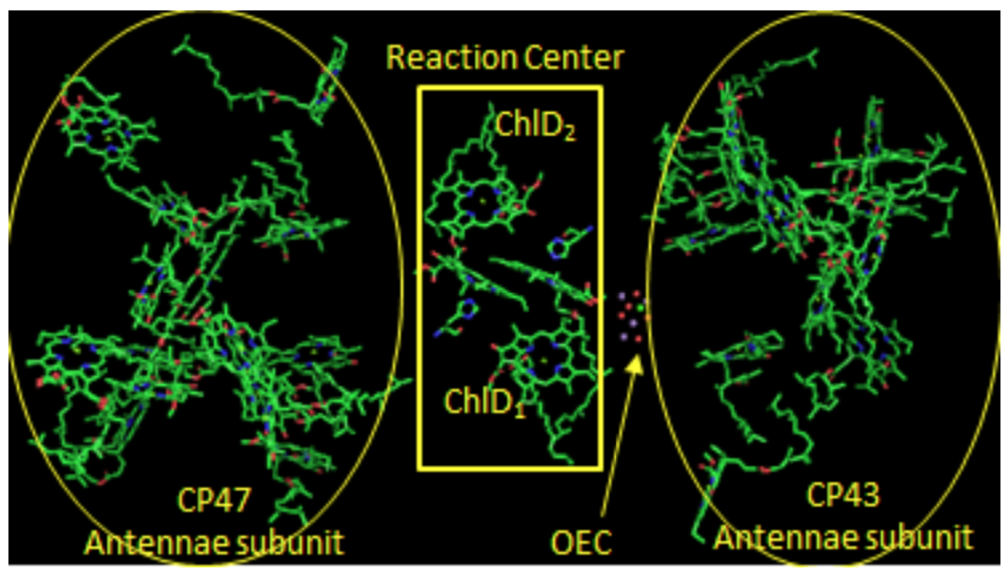
- What is the purpose of all those other chlorophylls and carotenes near the reaction center?
- What is the likely mechanism through which energy gets to the reaction center? Circle one.
Fluorescence | Phosphorescence | Radiationless Relaxation
Each pair of chlorophylls (ChlD1/ChlD2 & PD1/PD2) acts together as an “excitonic dimer”, called P680, referring to the dimer’s absorption maximum.
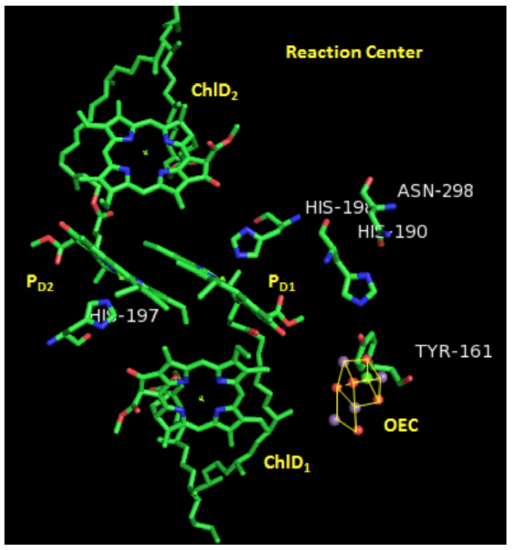
- Describe the relative orientation of two chlorphylls within one P680 dimer.
- Describe the relative orientation of one dimer with respect to the other.
- Suggest a reason for this orientation.
Photosystem II: Radiationless Energy Transfers in chlorophylls
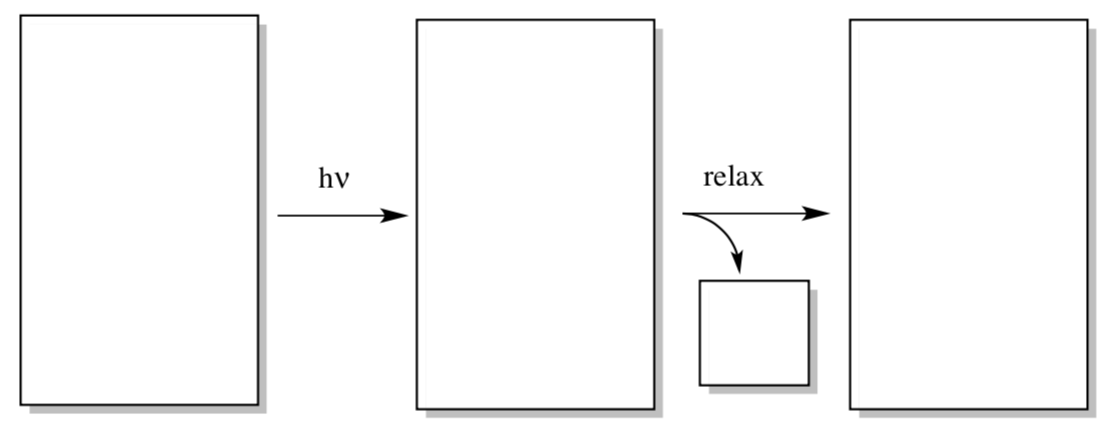
Let’s examine how energy could be transferred into the reaction center from chlorophyll. In the first step of the reaction, an electron is excited from the ground state to an excited state somewhere in the antennae region.
- Draw a generic MO diagram for this process. That’s right; you need a HOMO, a LUMO, an energy scale, and a couple of electrons in both your before and after picture.
- What else is produced when the molecule relaxes again?
Excitation of molecule “a” can lead to excitation of nearby molecule “b” via radiationless energy transfer.
- Show this process in the diagram below.
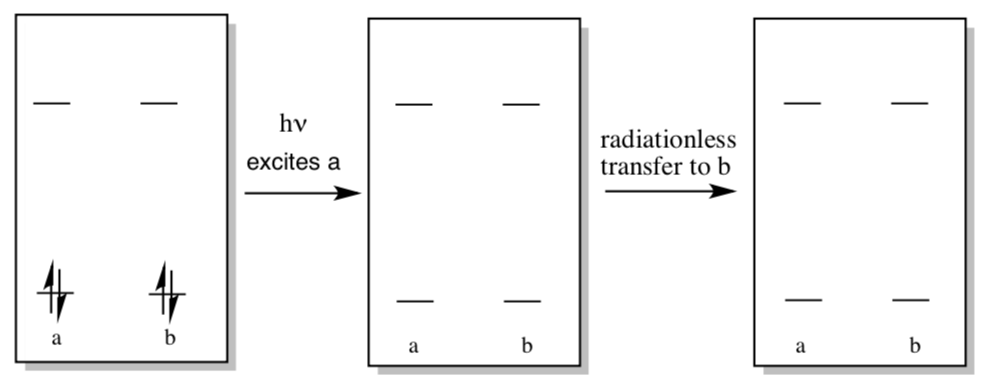
- How might this process lead to high efficiency excitation of reaction center chlorophylls?
Now we will consider the key process: how the reaction center chlorophyll becomes nature’s most powerful oxidant.
Photosystem II: Reaction Center Electron Transfers in P600
Electron / hole pair formalisms are an essential tool in understanding photoredox chemistry. In the first step of the reaction, an electron is excited from the ground state to an excited state.
- Draw a generic MO diagram for this process. That’s right; you need a HOMO, a LUMO, an energy scale, and a couple of electrons in both your before and after picture.
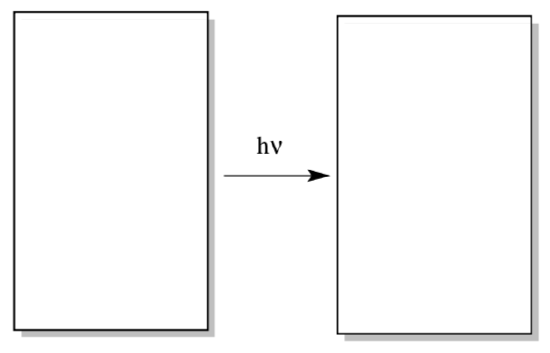
- Label the excited electron.
- Label the hole (the place where an electron used to be).
- The excited electron acts as an oxidizing agent / reducing agent. (circle one)
- The hole acts as an oxidizing agent / reducing agent. (circle one)
Now imagine a molecule that is going to get reduced.
- An electron will be added / removed. (circle one)
- Draw a generic MO diagram for this process.
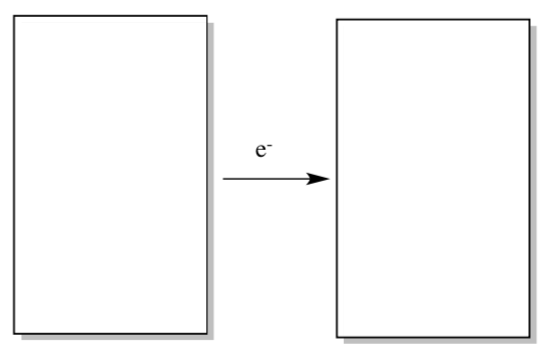
Photosystem II: Reaction Center Electron Transfers in P600
You have seen many examples of how thermodynamic properties and energy levels depend on the environment. Suppose you have two identical, neutral molecules in two different environments.
- Fill in the amino acid residues as indicated.
- Which molecule would be reduced more easily?
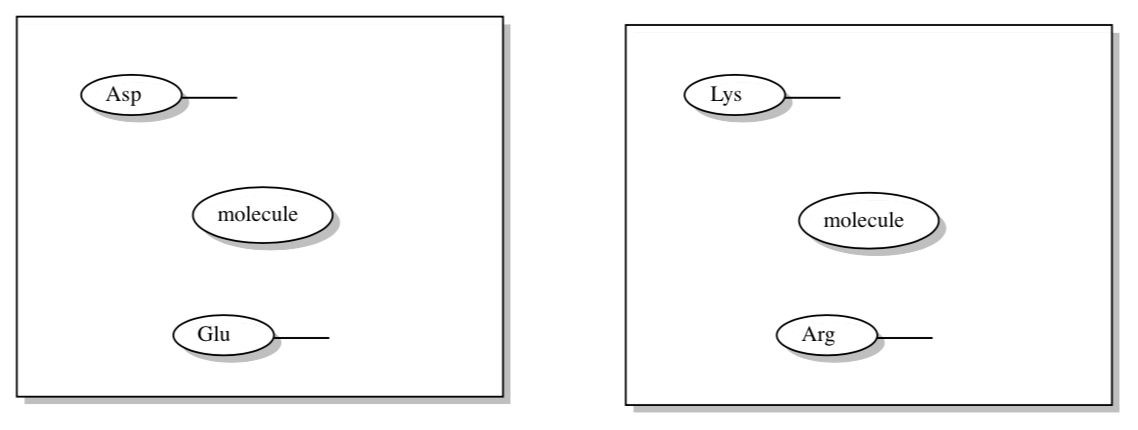
Assume molecule b has a LUMO that is slightly stabilized by its environment. Instead of radiationless energy transfer, we may get single electron transfer between two molecules (excitonic dimers).
- Complete the MO diagram to show the electron transfer in the excitonic dimer.
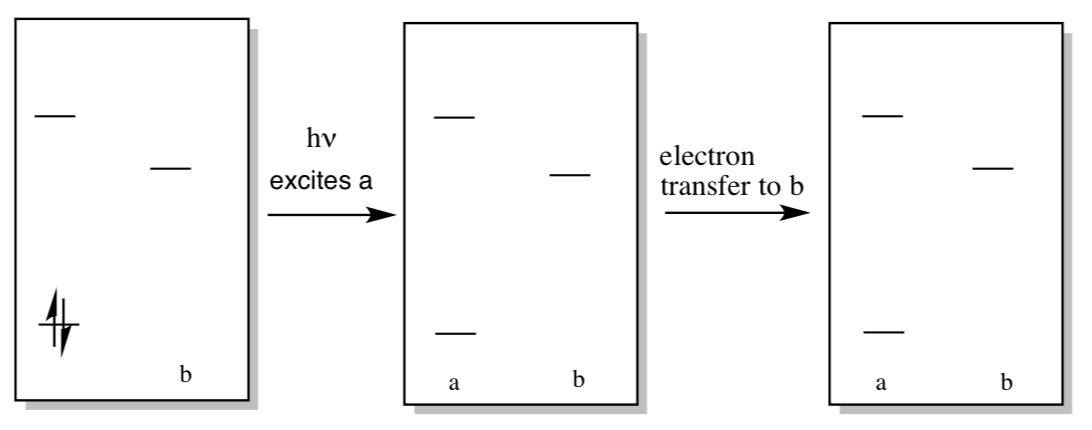
- In the box on the right, label either a or b as the reducing agent (molecule with excited electron).
- In the box on the right, label the oxidizing agent (molecule with hole).
- What would be the charge on molecule “a”? Molecule “b”?
Photosystem II: Reaction Center Electron Transfers in P600
The chlorophyll pair in P680 is an excitonic dimer as shown on the previous page.
- Complete the structures of the excited state P680* to reflect electron / hole separation and the appropriate electronic transition.
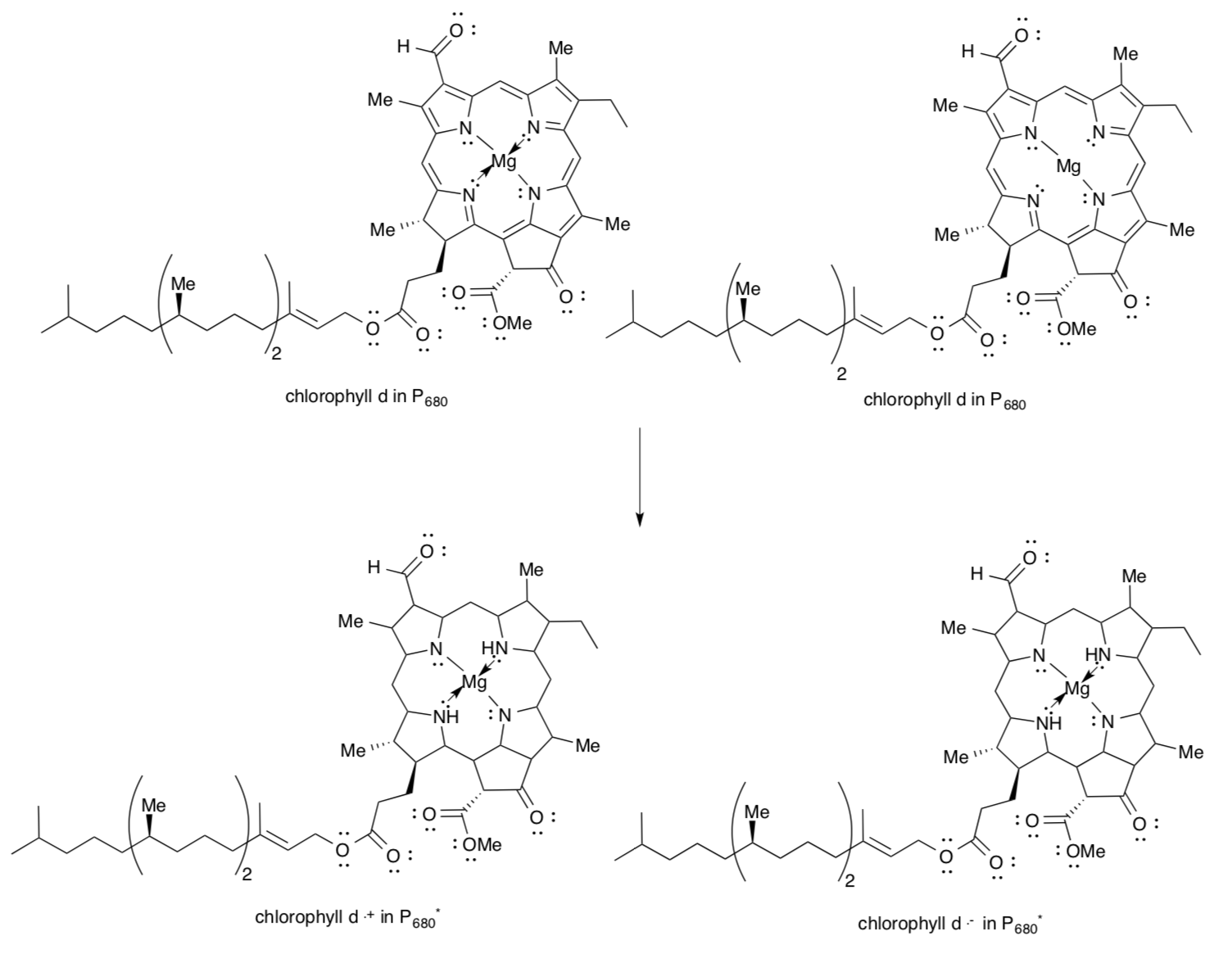
In the drawing on the left, assume the lower ChlD1 corresponds to molecule a, whereas the upper ChlD2 corresponds to molecule b.
- Place a (+) and a (-) beside the picture to indicate charge separation within the dimer upon excitation.
- ChlD1* will be an oxidant / reductant. (circle one)
- ChlD2* will be an oxidant / reductant. (circle one)
Photosystem II: Electron Transfer Energetics
The excited electron and the “hole” left behind must be separated so they don’t just recombine.
In the crystal structure on the previous page, you can see a cluster of atoms called OEC. OEC is the site where water is going to be oxidized.
- Comment on how the proximity of ChlD1 to OEC will affect water oxidation.
Photosystem II: The Oxygen-Evolving Complex
The Oxygen Evolving Complex (OEC) is a cluster of metals that removes the electrons from H2O and transfers them to Photosystem II. The “Kok Cycle” shown below.
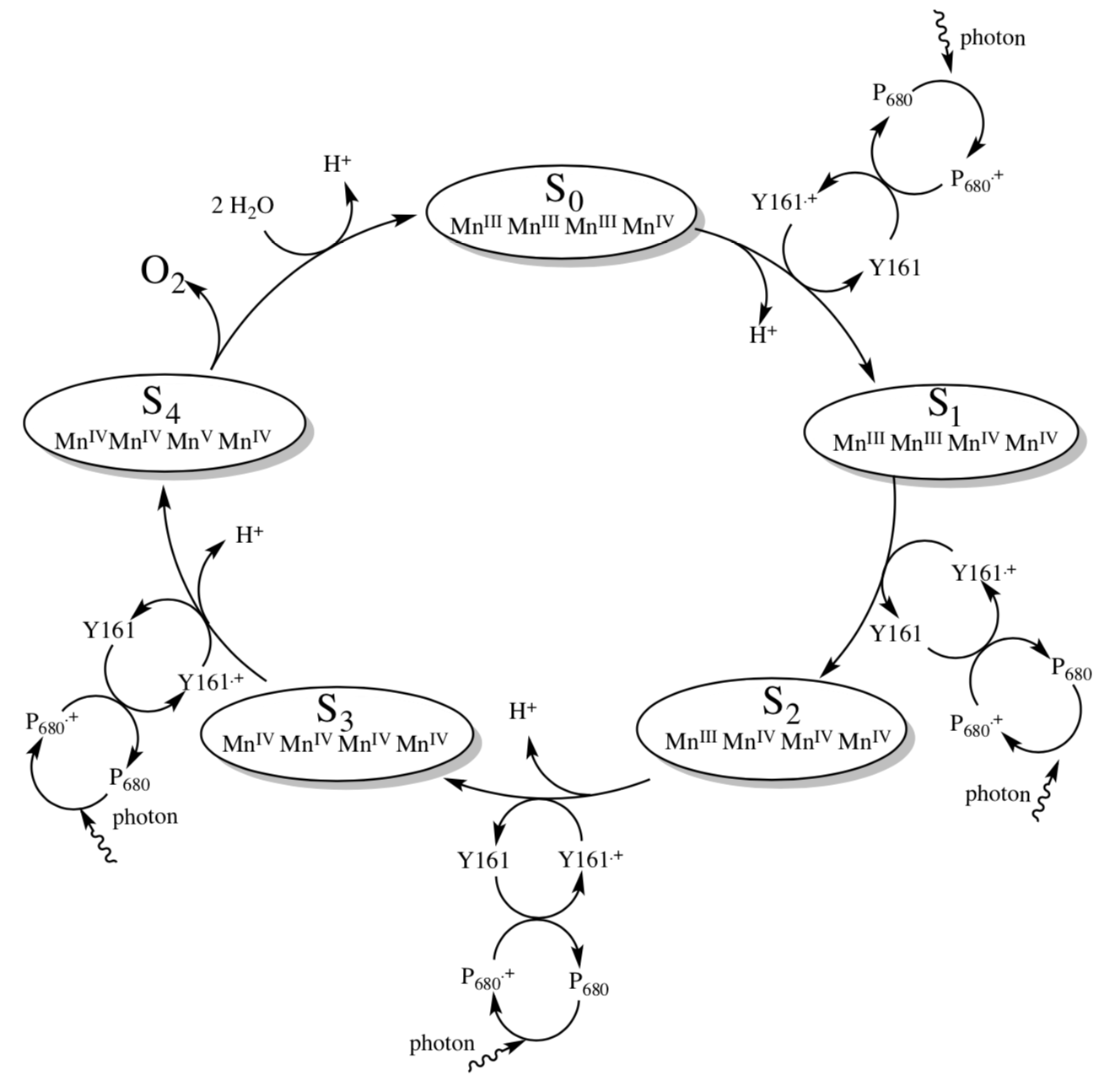
The reduction potential of P680+ is 1.30 V while the Eredo for O2 -> H2O is 0.816 V.
- Can P680+ oxidize water? What is the overall potential for the reaction?
- How many P680+ will be needed to convert water to O2? ______
- How many photons must be absorbed to produce those P680+? ________
- At what point in the cycle is O2 evolved, in terms of the oxidation states of the manganese cluster?
- Why do you think the oxygen is evolved at that point (and not earlier)?
In order for the cycle to be catalytic, the missing electron from P680+ must be replaced. It is initially replaced by an electron from tyrosine (Y161).
- Show why tyrosine is a good electron donor.
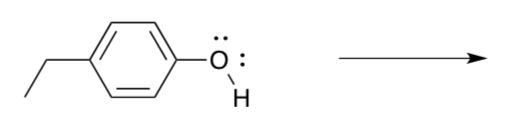
Remember that P680+ has enough oxidizing power to affect the conversion of water to O2, based on thermodynamics. Why bother with the intermediaries?
- Suppose P680+ (or Y161) reacted with a water molecule directly. Show the products of the first electron transfer.
- The result is a free hydroxyl radical. Explain why that might not be a good thing.
- How many water molecules would we have to convert to hydroxyl radicals before we started making an O-O bond?
Clearly, the problem is making sure two water molecules get to the same place to get oxidized in short succession. That way, we avoid generating free reactive oxygen species.
- Explain how a cluster of metal ions might help bring water molecules together.
Manganese Oxo Cluster of the OEC
A crystal structure of the OEC established that there was a “cube” of three Mn ions and a Ca ion, with oxygens in the opposite corners of the cube.
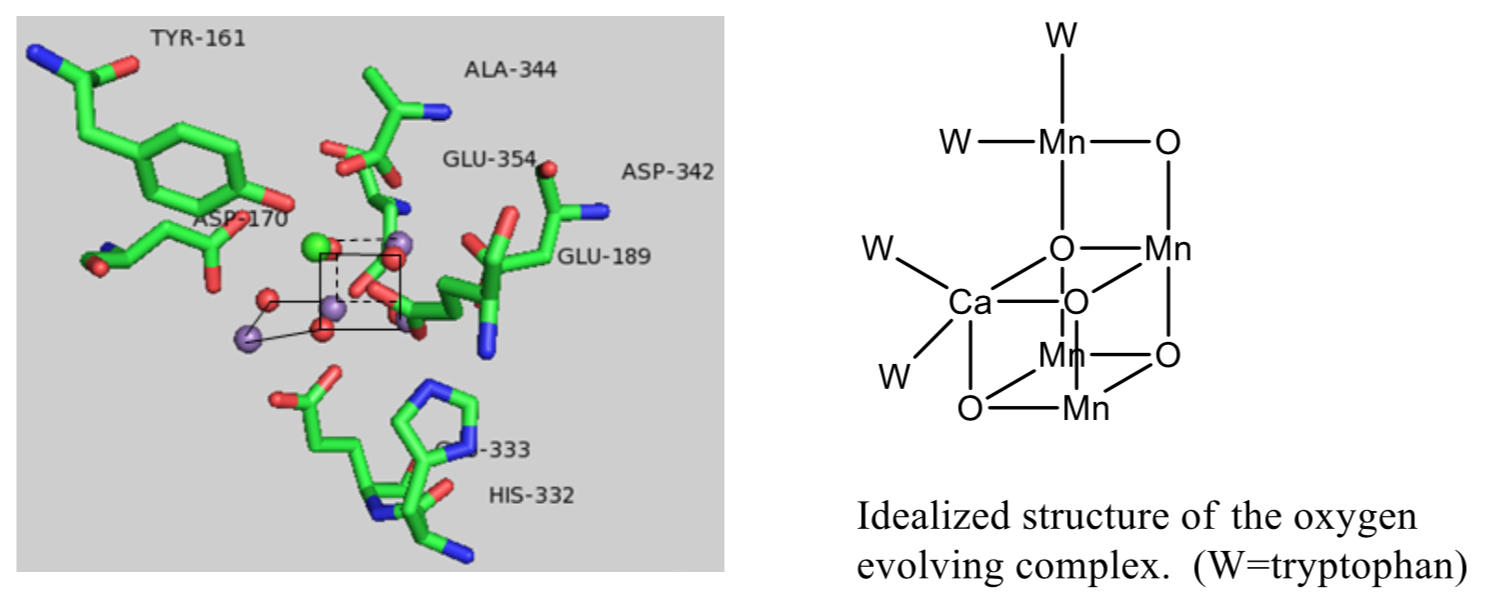
- Ca ions typically only have one oxidation state (Ca2+) in the S0-S4 states. Why?
- The metals ions in this cluster come from the middle of the transition metal block. Why?
- Why do you think there are multiple metal ions involved the cluster, and not just one that goes from M(X) to M(X+4)?
The assignment of oxidation states is not definitive for the So complex. However, there is ample evidence that the S1 state is Mn(III)2,Mn(IV)2. Assume the coordination geometry is octahedral for all Mn.
- Draw the d orbital splitting diagram for each oxidation state (III & IV). Place the proper number of electrons in the diagram.
- Which of the S states must have odd numbers of electrons?
Odd numbers of electrons are significant because such species are easier to detect spectroscopically.
One possibility is that S3 has a Mn(V)=O linkage (terminal oxo ligand.) Study of the (salen)Mn complex below showed that it could be oxidized to produce Mn(V)=O in addition to the four donor atoms of the ligand.
- Modify the structure to fit the description. How many unpaired electrons would it have?
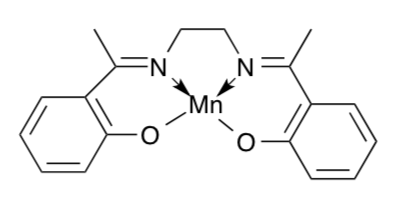
Further study of [(salen)Mn=O] revealed that the Mn=O bond was unusually short. The authors proposed that there was some triple bond character to the bond.
- Draw this proposed structure and calculate the formal charge on the O in this resonance structure.
The role of the Ca is thought to involve binding of H2O. Binding of H2O increases the acidity of the water molecule.
- Explain why. As such, a bound water molecule could be viewed as a source of OH-.
We still haven’t explained how a one-oxygen species like H2O can link to another O to form O2.
- Propose a mechanism for this based on a Mn(V)oxo ligand and a Ca-bound water molecule.
Mechanism of the OEC
- Use the simplified structure below (only two Mn instead of four) to complete a summary of the Kok cycle and produce O2 from water.
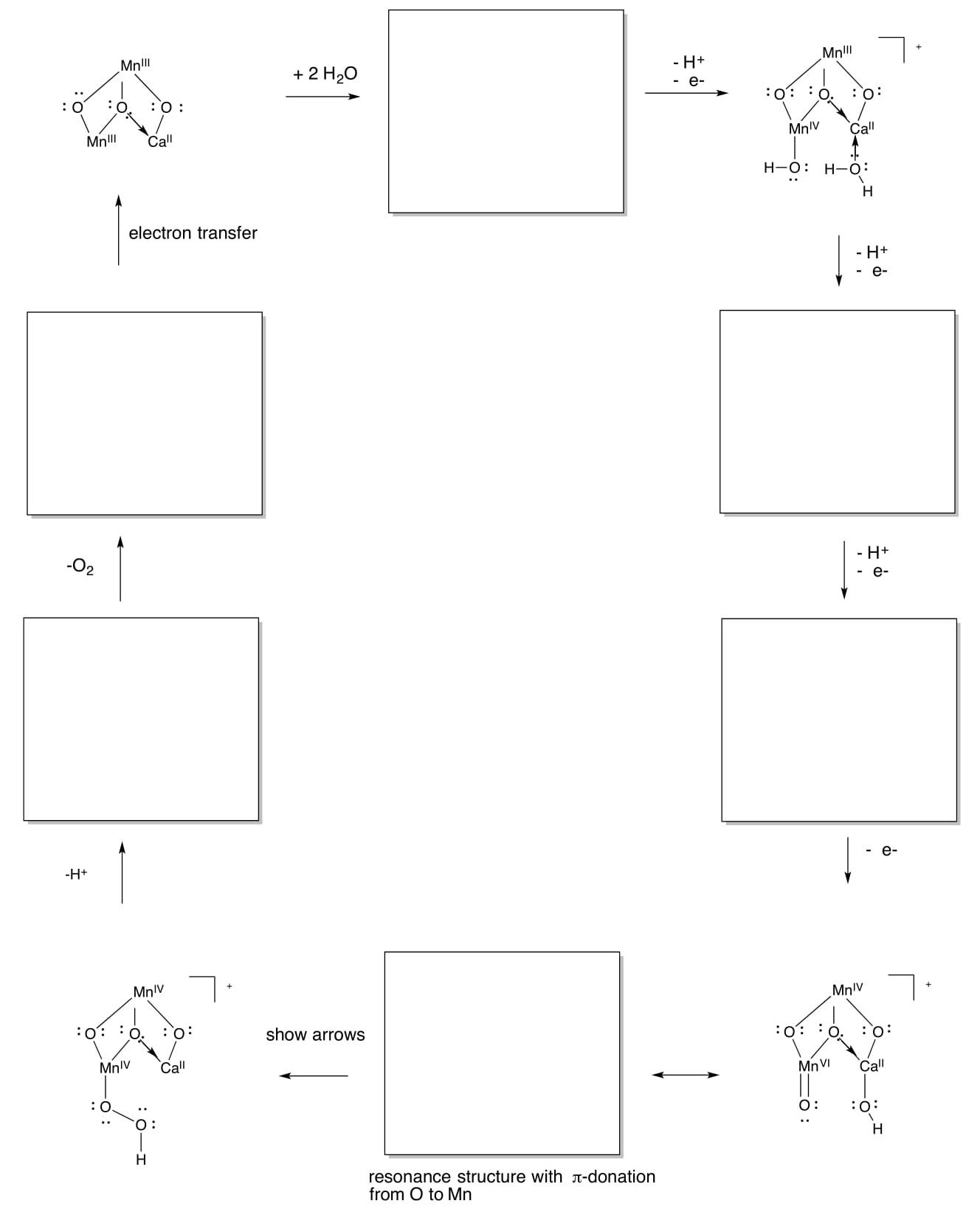
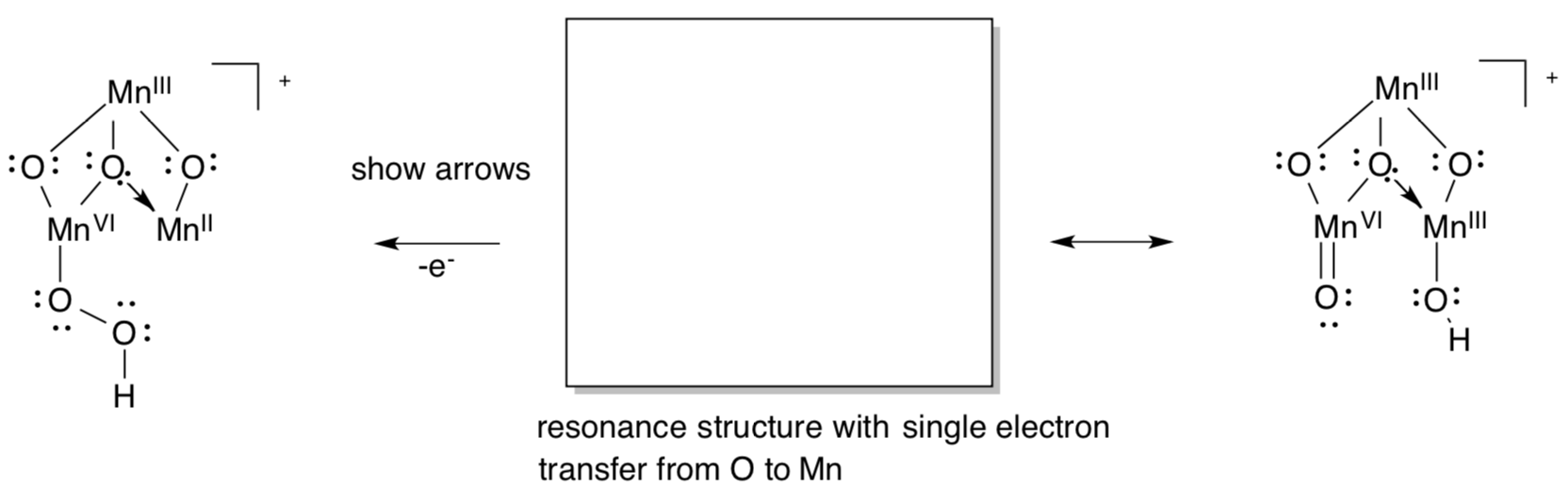
Alternate Mechanism of the OEC
Other research suggests an ancillary role for the calcium, with the two water molecules binding only to Mn.
- Show how the redox activity of Mn could make another mechanism possible for O-O bond formation.
Photsystem II and the Electron Transport Chain
The excited electron and the “hole” left behind in P680* must be separated so they don’t just recombine. The hole takes part in the Kok cycle. The excited electron is transferred to a pheophytin molecule to form Pheo-.
- Fill in the structure for the reductant in P680*. Your drawing should reflect electron/hole separation from an earlier page.
- Show the structures of chlorophyll d and pheophytin after electron transfer.
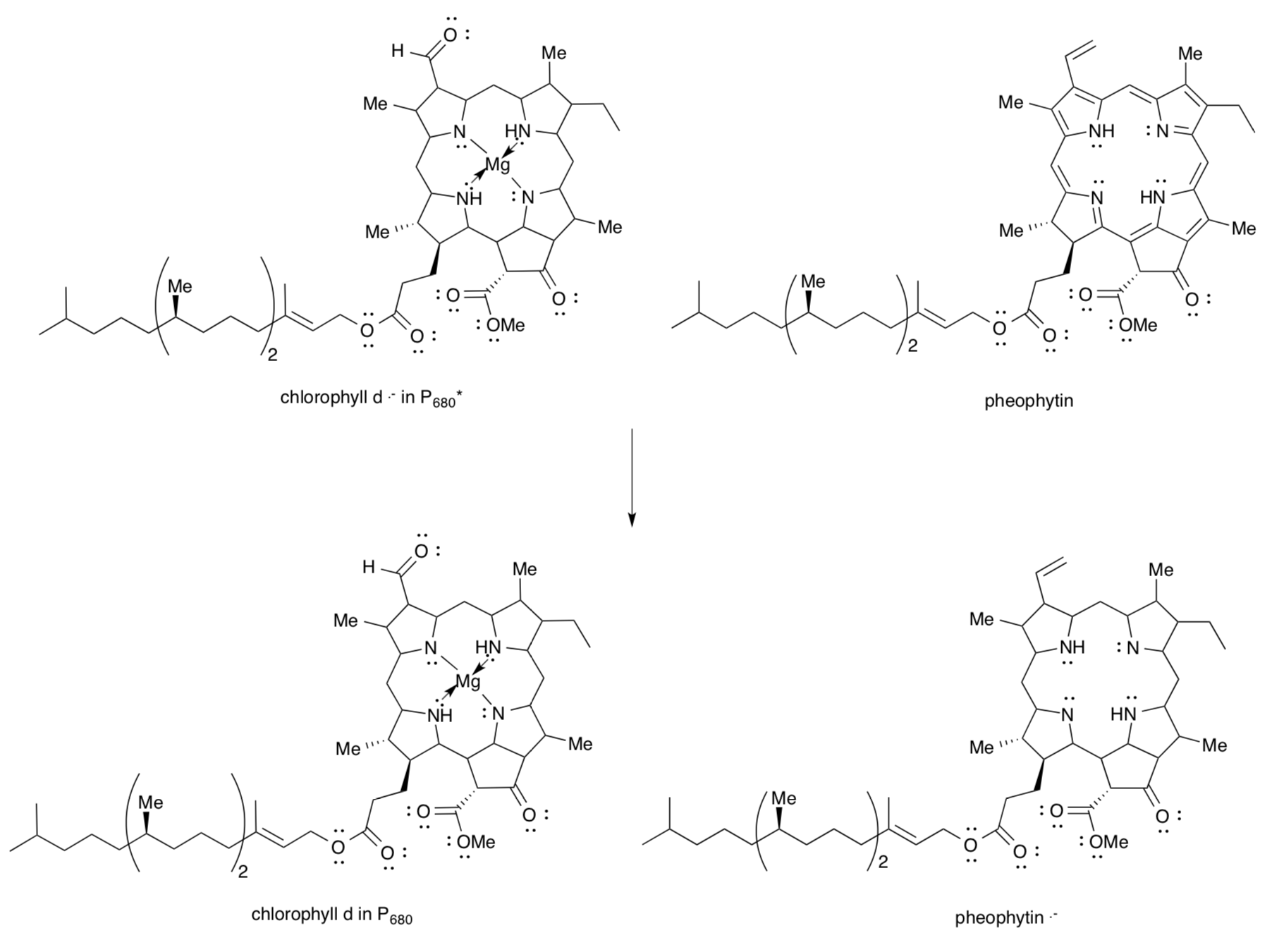
- Pheophytin . - is an oxidizing agent / reducing agent. (circle one)
Pheophytin transfers the electron to a plastoquinone (PQ). These cofactors are shown below.

- Provide products of the reduction using the template structures below.
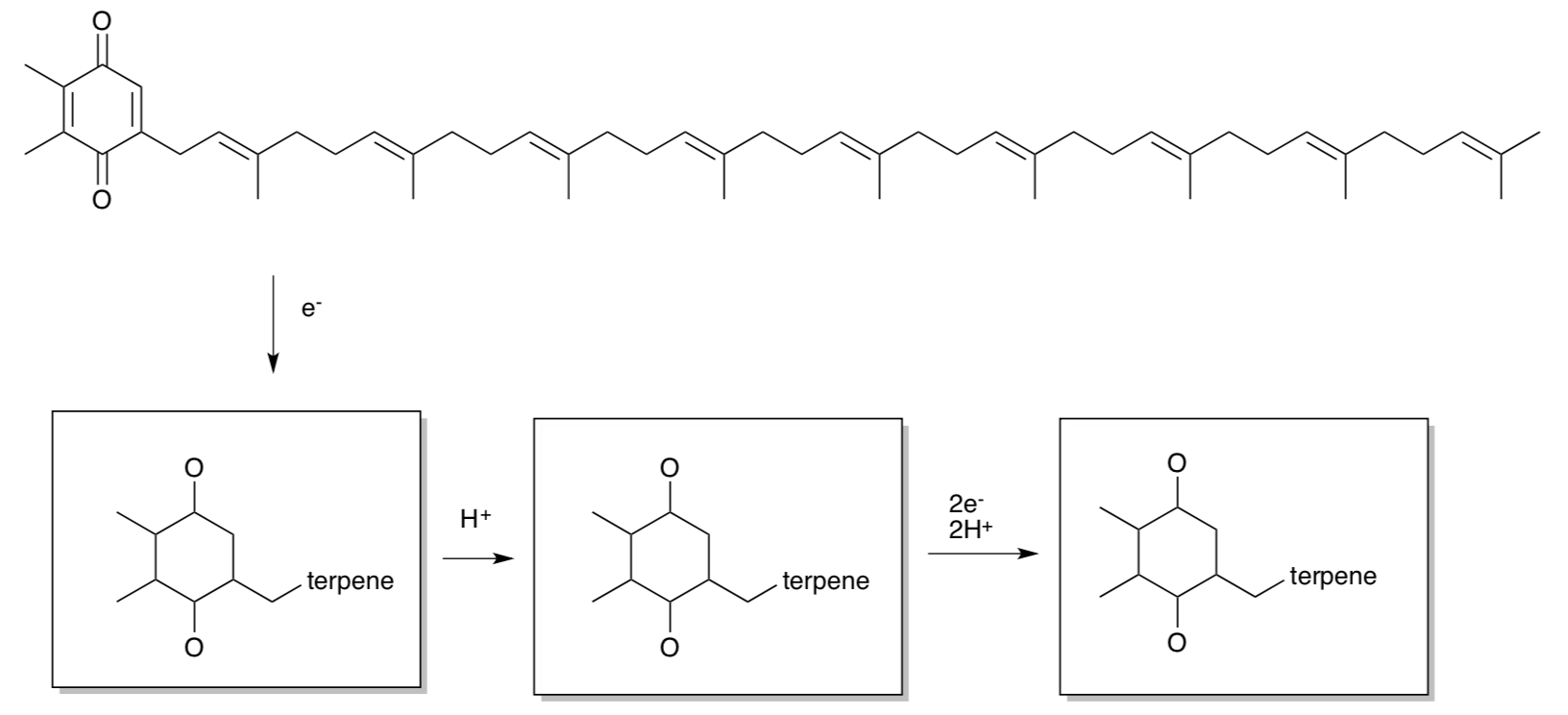
- Is PQ water-soluble or membrane-soluble? Why?
PQ delivers the electron to Cytochrome b6f in order to continue the electron transport chain.
Photosynthesis: Proton Pumping in Photosystem II
Protons produced during the Kok cycle must move away from the complex and toward the lumen where they would contribute to the development of a proton gradient. X-ray structures reveal a number of water molecules in the vicinity of the OEC.
- Add curved arrows to illustrate a possible sequence of proton transfer reactions.

An expanded view of the OEC and part of the protein is shown below.
- Circle the residues expected to take part in proton transfer.
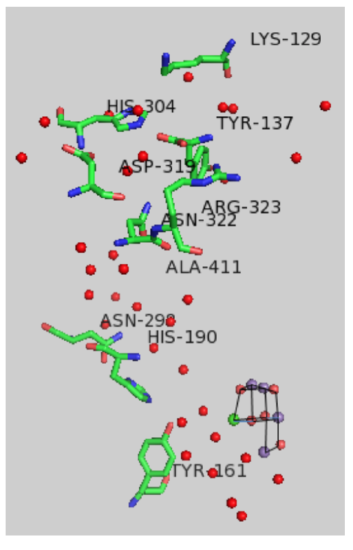
- In addition, amino acids with non-polar residues could act as bases via: [ the carbonyl oxygen / the amide nitrogen] (circle one)
Summary
The figures below show how PSII interacts with the other membrane complexes to produce O2, NADPH, and ATP. The bottom figure shows a graph of standard reduction potentials for key players. It is from this diagram that the name Z scheme arises.
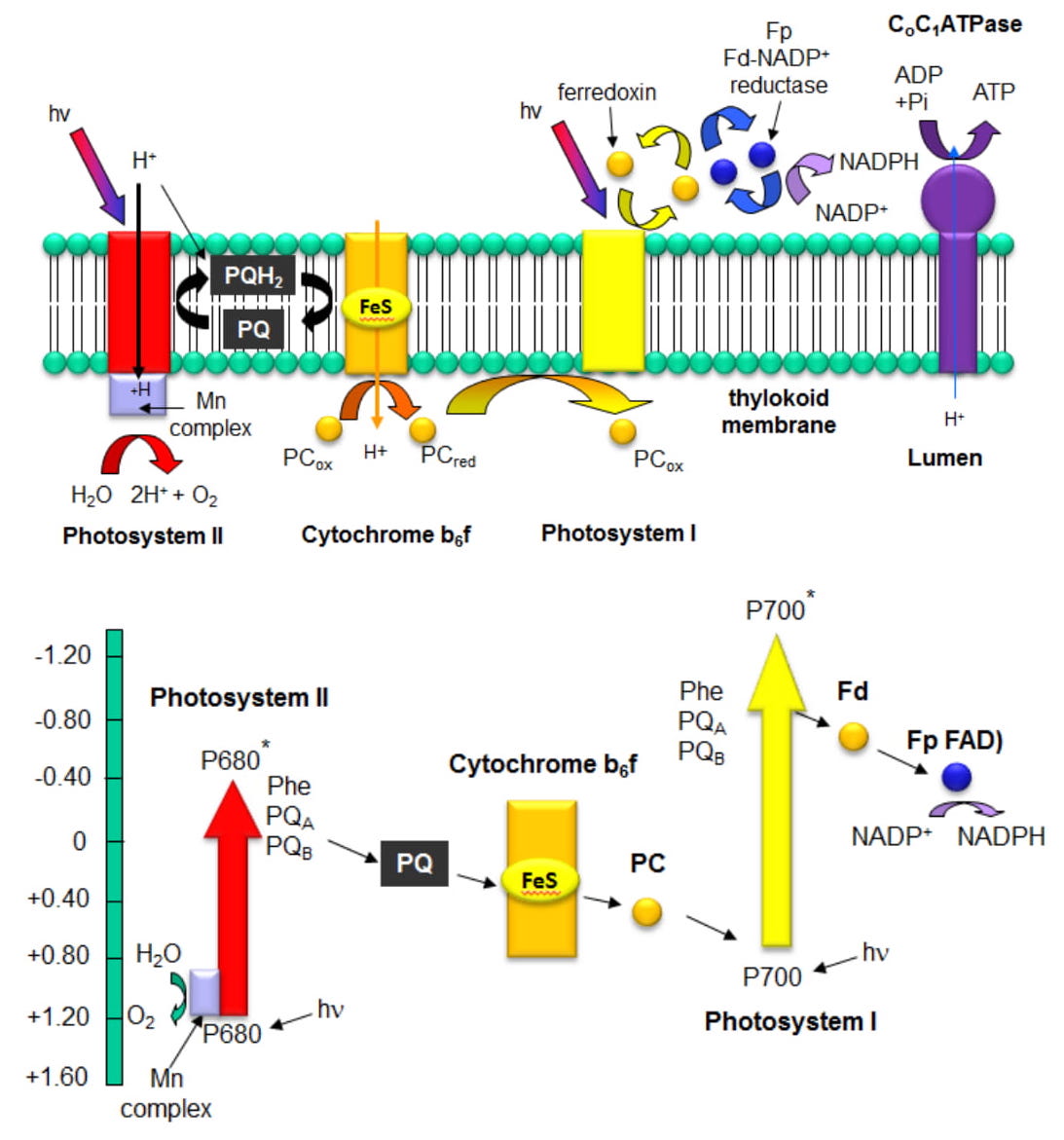
References:
- Chem. Rev. 2014, 114, 4175−4205
- Nature (2015) 517, 99–103
- Annu. Rev. Biochem. 2013. 82:577–606
- Current Opinion in Chemical Biology (2012) 16, 11–18.
- Journal of Biophysical Chemistry (2012) 3, 111-126.
- Coordination Chemistry Reviews (2012) 256, 2445– 2452
Schematics for Photosynthesis:
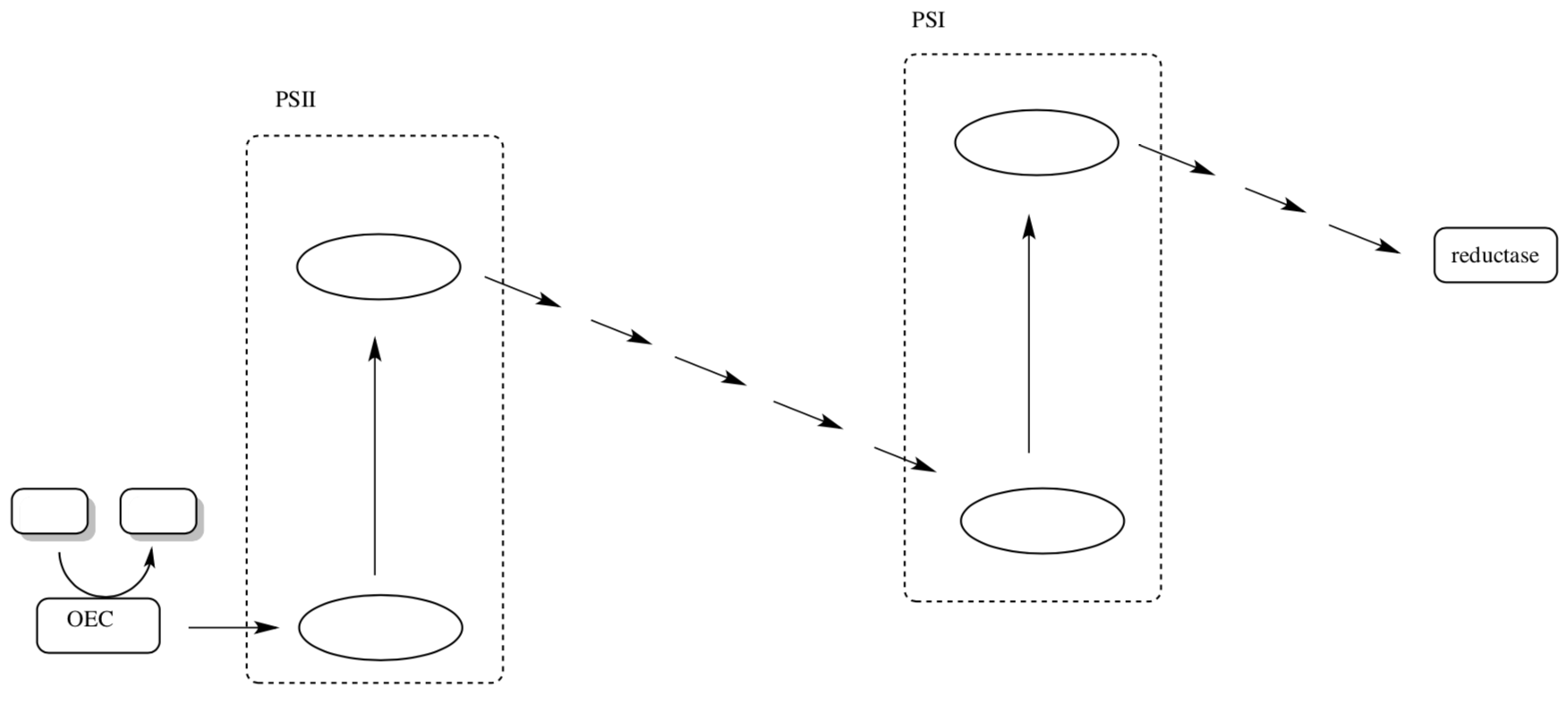
- Complete the following picture of the Z scheme for photosynthesis with the following pieces of information:
- Indicate which steps require light.
- Label at least 4 of the mobile electron carriers.
- Indicate the reaction completed by the OEC.
- Indicate the reaction carried out by the reductase.
- Label P680, P680*, P700, and P700*.
Application Problem
- Model Study for OEC
Much of our knowledge of molecules like the OEC in PS II come from the study of synthetic model compounds such as (salen)Mn and others. Another model for OEC is shown below. Upon oxidation, it does catalytically evolve O2.
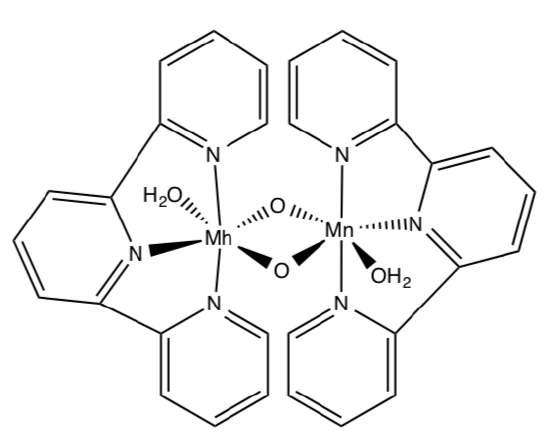
- Draw a mechanism for the process. (Add arrows and fill in missing intermediates).
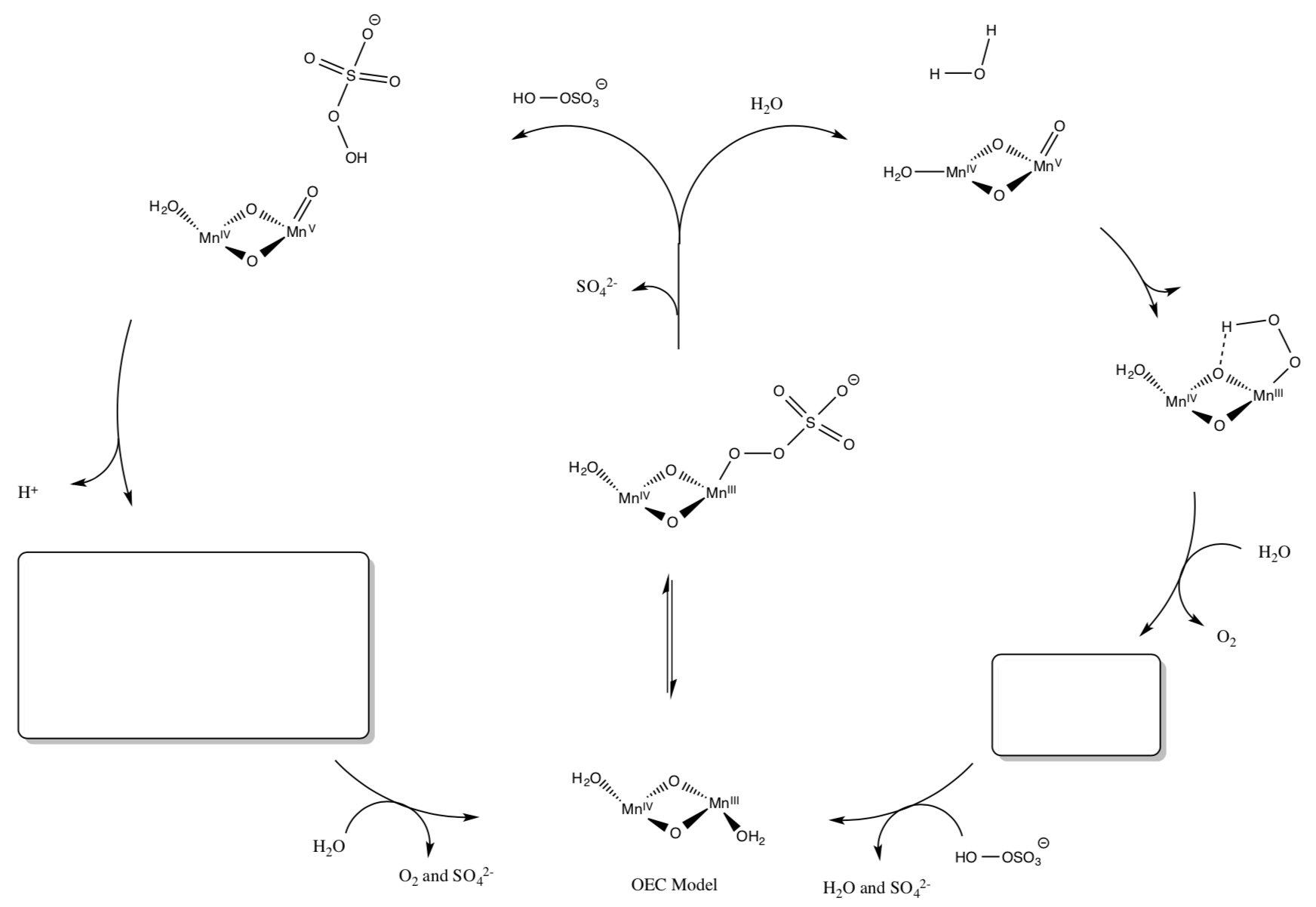
- Give a couple of advantages and disadvantages of studying models of active sites of enzymes (as opposed to studying the real thing.)
- Compare and contrast this model to the OEC.
- Draw a mechanism for the process. (Add arrows and fill in missing intermediates).
- Metal Organic Frameworks for Light Harvesting and Photocatalysis
Wang, Wang, & Lin, ACS Catalysis, 2012, 2, 2630-2640.
Modeled on photosynthesis, several research groups have investigated the use of visible-light photoredox catalysts to initiate organic transformations.
One reaction that scientists are particularly interested in is the formation of H2 gas. The hydrogen gas would be used for fuel.
- Write a reaction for the combustion of hydrogen.
- In contrast, burning hydrogen produces _________, which should have [less / more ] environmental impact than the combustion of methane or propane.
To form H2 gas from H+ ions.
- Write a half-reaction for this process.
- Using the reduction potential table, what is the reduction potential for this reaction?
- List two metals that would be capable of reducing H+ to form H2.
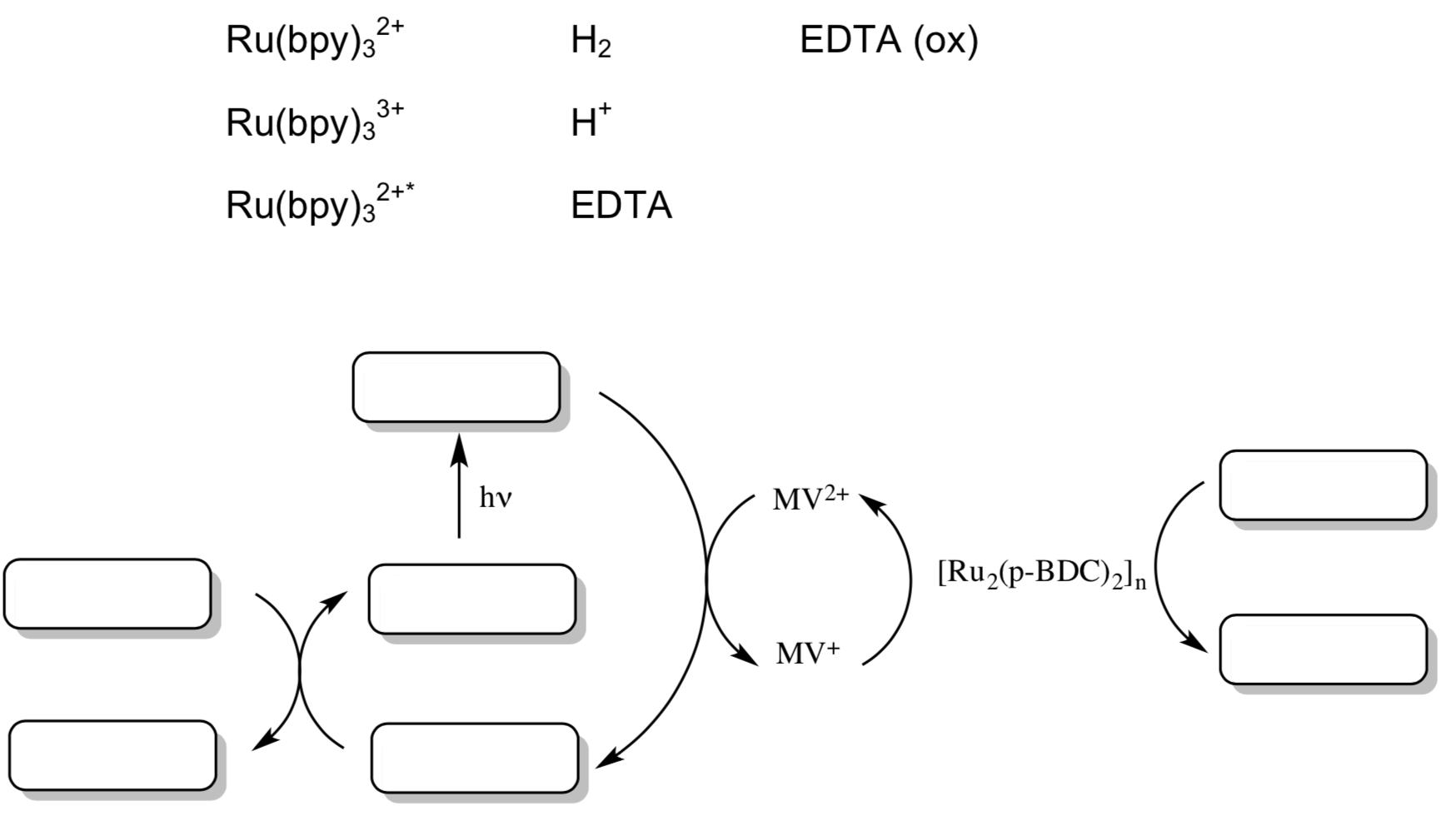
A porous MOF with the formula of [Ru2(p-BDC)2]n (BDC = benzenedicarboxylate) has been shown to be capable of visible-light driven proton reduction.
- Propose a catalytic cycle for this reaction assuming that electrons and protons are readily available.
- MOF for photocatalysis (cont)
Mori and co-workers found that a MOF was capable of visible-light driven proton reduction with this Ruthenium catalyst to donate electrons, Ru(bpy)32+ as a photosensitizer, MV2+ as an electron relay, and Na2EDTA as a sacrificial reductant.
- Add the following components to this schematic for photocatalysis.
These researchers also integrated chromophores into MOF structure that is wired to the charge separation center.
- Suggest a reason why the researchers set the chromophores in a relay system like this.
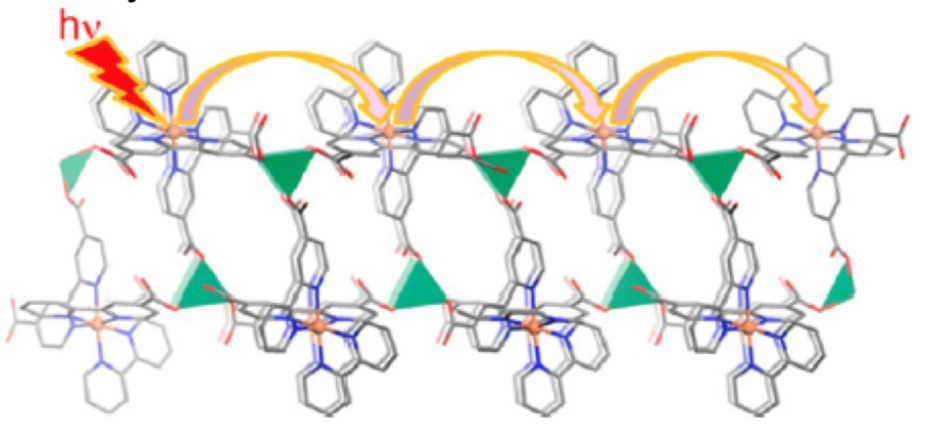
- Add the following components to this schematic for photocatalysis.


