1: Acetylation of Aniline (Experiment)
- Page ID
- 126789
Acetanilide is an analgesic, which was formally known as Antifebrin1, and is structurally similar to acetaminophen (or Tylenol). However, unlike acetaminophen, acetanilide is toxic. Acetanilide is prepared from aniline using an acetylation reaction. Acetylation is often used to place an acetyl protecting group on primary or secondary amines to reduce their reactivity toward oxidizing agents or electrophiles. Acetamides are usually crystalline solids which can be a help in purification by recrystallization. The melting points can be used for characterization and identification of the corresponding compounds.
1. Cahn, A; Hepp, P. (1886), “Das Antifebrin, ein neues Fiebermittel”, Centralbl. Klin. Med. 1886, 7, 561-65
Reaction
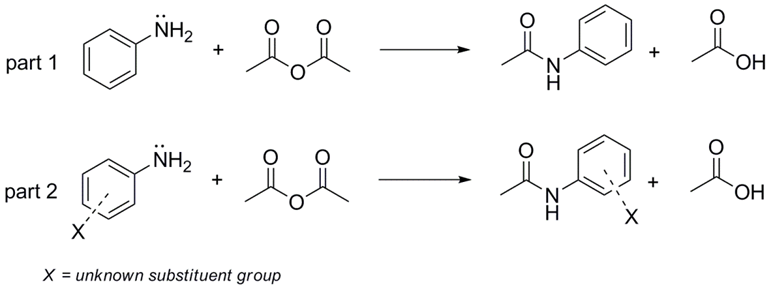
Acetylation of aniline and unknown substituted anilines with acetic anhydride
Techniques
- the Beilstein test
- melting point
- recrystallization
- gravity & vacuum filtration
- thin layer chromatography
- and infrared spectroscopy.
Objective
In part 1 you will convert aniline to acetanilide using an acetylation reaction described below. In part 2 you will be given an unknown aromatic amine from table 1-1 below. The amine will be converted into its acetamide analog also using the acetylation procedure described below. Your objective is to compare the two reactions and determine the identity of the unknown amine/acetamide product.
Procedure
Reagents:
- Aniline
- HCl (concentrated HCl is 37% w/w)
- Acetic anhydride
- Sodium acetate
Part 1
Dissolve 500 mg of aniline in 14 mL of water. Note that aniline is immiscible in water and two layers should be observed. Add 0.45 mL of concentrated hydrochloric acid. Measure out 0.6 mL of acetic anhydride and prepare a solution of 530 mg of sodium acetate in 3 mL of water. Add the acetic anhydride to the solution of aniline hydrochloride in water, mix by swirling, and immediately add the sodium acetate solution. The solution becomes white as acetanilide precipitates. Cool the solution in an ice bath and collect the solid acetanilide by vacuum filtration. Recrystallize from 95% ethanol - it may be necessary to use a small amount of water.
Part 2
Use the above procedure for acetylation of the substituted unknown aniline. Exact quantities cannot be calculated since the starting material is an unknown.
Characterization
Both products of part 1 and 2 should be characterized by TLC. Each product should be co-spotted against the starting material for each reaction, respectively, and against each other. Collect the Infrared spectrum of the part 1 product, acetanilide, and the unknown starting material and product from part 2. Characterize the products of part 1 and 2 by m.p and using the Beilstein test for halogens.
For the Beilstein test first clean a copper wire by holding it briefly in a flame. Touch the wire to a sample of the compound and return it to the flame. A blue-green color in the flame indicates the presence of a halogen. It is a good idea to test the procedure on a compound known to contain a halogen before trying it on your own compound.
Use Reaxsys or SDBS to find literature IR spectra of aniline, acetanilide, unknown subtituted amine and its corresponding acetamide product for comparison to the spectra that you obtain in lab.
Post-lab Questions
Write your answer at the end of your lab notebook pages for this experiment:
- Describe in your own words what happens to aniline as concentrated hydrochloric acid is added.
- Why is sodium acetate used? What if NaOH was used instead?
- Compare the behavior of the unknown substituted amine with aniline in the acetylation reaction. How did the 2 reactions differ? What how might the structural differences in the unknown substituted amine cause it to react differently than aniline, which is un-substituted?
- How does the Infrared spectrum of your unknown amine compare to that of aniline? How does the spectrum of your unknown acetamide product compare to that of acetanilide?
| Amine | bp (°C) | mp (°C) | Acetamide mp (°C) |
|---|---|---|---|
| aniline | 184 | -- | 114 |
| N-methylaniline | 196 | -- | 102 |
| ortho-toluidine | 200 | -- | 110 |
| meta-toluidine | 203 | -- | 65 |
| 2-chloroaniline | 209 | -- | 87 |
| 2-ethylaniline | 210 | -- | 111 |
| 2,5-dimethylaniline | 213 | 14 | 139 |
| 2,6-dimethulaniline | 215 | 11 | 177 |
| 4-ethylaniline | 216 | -6 | 94 |
| 2,4-dimethlaniline | 217 | -- | 133 |
| 3,5-dimethylaniline | 220 | 10 | 144 |
| 2,3-dimethylaniline | 221 | 4 | 135 |
| 3-chloroaniline | 230 | -- | 72, 78 |
| meta-anisidine | 251 | -- | 81 |
| para-toluidine | 200 | 44 | 147, 153 |
| 3,4-dimethylaniline | 224 | 49 | 99 |
| 2,5-dichloroaniline | 251 | 50 | 132 |
| para-anisidine | 240 | 58 | 127 |
| para-bromoaniline | 245 | 66 | 168 |
| para-chloroaniline | 232 | 72 | 172, 179 |


