21.4: Experiment 65 Reactions
- Page ID
- 214016
Experiment 65 uses the reaction between acetic anhydride and vanillin to illustrate the effects of catalysis (as shown above) and the reactivities of the functional groups present in the molecules. In order to figure out the puzzle posed by part B of the experiment, we may want to keep the following in mind.
1. When an anhydride reacts with an alcohol (either under acidic or basic conditions), the anhydride plays the part of the electrophile, undergoing nucleophilic attack by the alcohol.
2. The vanillin molecule has several functional groups, among them alcohol, ether, and aldehyde. When it reacts with acetic anhydride we can predict a reaction pattern similar to the examples given above, with vanillin acting as the alcohol (nucleophile) and the anhydride acting as the electrophile. By working out the mechanism you should be able to arrive at the structure of the esterification product obtained from the acid and the base catalyzed reactions.
3. The base-catalyzed reaction is a straightforward esterification and doesn’t produce any other significant products. In other words, the esterification stops at the ester. YOUR LAB REPORT SHOULD INCLUDE THE STRUCTURE OF THIS ESTER (product of base catalysis) AND SUPPORTING NMR SPECTRUM (with peak assignments).
4. However, the acid-catalyzed esterification does not form only the ester. It forms the ester, but this ester continues to react because there are other functional groups present in the molecule that can react with the anhydride under these conditions.
5. The ether functional group (CH3O) attached to the benzene ring, and the benzene ring itself, are not reactive under these conditions, and therefore do not undergo any kind of modification.
6. That leaves us with the aldehyde, which is known to be one of the most reactive functional groups in organic chemistry. How does it react? The presence of the carbonyl group (C=O) basically sets the tone for aldehyde chemistry. The polarization of the C=O makes the carbon highly electrophilic. Therefore we need a nucleophile.
7. We only have one other molecule present in the system, namely acetic anhydride. Can it act as a nucleophile? The answer is yes, due to the presence of unshared electron pairs on the three oxygen atoms. So the question becomes, are all the oxygen atoms equally nucleophilic, or is one of them more nucleophilic than the others? Let’s look at the resonance structures of the anhydride to predict the site of the most nucleophilc oxygen.
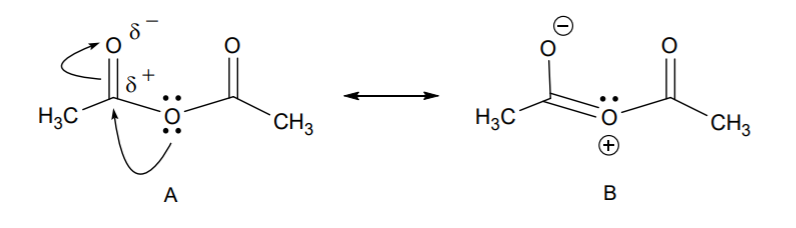
8. Resonance structure B reveals that the carbonyl sp2 oxygen is more nucleophilic than the central sp3 oxygen. So we can use structure B in a nucleophilic attack on the aldehyde. Let’s see how this works in the acid-catalyzed reaction.
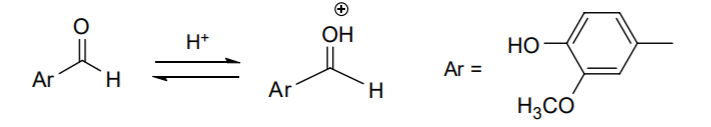
9. Under acid catalysis, the aldehyde oxygen becomes protonated first, leaving the sp2 carbon more susceptible to nucleophilic attack:
This is then followed by nucleophilic attack from resonance structure B on that carbon:
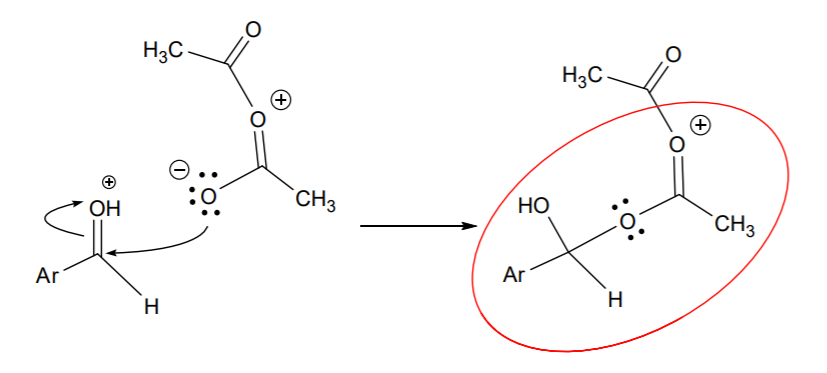
The positive charge shown on the central oxygen makes the circled part a good leaving group relative to the rest of the structure. Attack from the alcohol group on the sp2 carbon next to the positive charge results in internal displacement of the leaving group:

10. Loss of a proton from the resulting structure above leads to formation of the final product. Remember, this product contains an ester group that results from reaction between the alcohol part of vanillin and acetic anhydride. It also contains the product resulting from the process shown above. Therefore:
11. YOUR LAB REPORT SHOULD INCLUDE THE STRUCTURE OF THE PRODUCT FROM ACID CATALYSIS, DISCUSSED IN TERMS OF TWO THINGS:
a) the NMR spectrum obtained, with as many peaks assigned as possible, and
b) the paper by Kochlar et. al. (Conversion of aldehydes into geminal diacetates) 1
GOOD LUCK!
1 Kochlar, S.K. i., 48, 1765 – 1767 (1983). Please locate and obtain this paper using SciFinder, as learned before in the library instruction session.


