17.3: The Sulfonic Acid Group and its Derivative
- Page ID
- 213825
Sulfonic acids are organic analogs of sulfuric acid, an inorganic compound that is a very strong acid. Like sulfuric acid, sulfonic acids are highly corrosive, they react vigorously with water, and can cause skin burns.
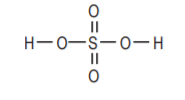
Sulfuric acid, H2SO4. Strong acid, highly corrosive.
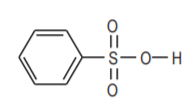
Benzene sulfonic acid, an organicanalog of sulfuric acid.
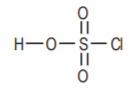
Chlorosulfonicacid, also a strong acid.
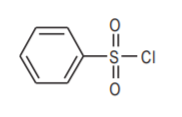
Benzenesulfonyl chloride. An organic analog of chlorosulfonic acid.

The amide functional group.
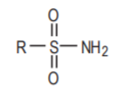
The sulfonamide functional group.
Because they are highly reactive towards water, some of these substances are also lachrymators. They react with water in the eye to produce hydrochloric acid (HCl), another corrosive and irritant substance.
HANDLE ALL OF THESE SUBSTANCES WITH CARE AND IN THE HOOD. ALWAYS WEAR GLOVES AND SAFETY GLASSES.
Sulfonyl chlorides contain a good leaving group (Cl). That is what makes them highly reactive towards water and other nucleophiles such as ammonia (NH3). These reactions are used in this synthesis, but they can also cause problems. For example, the second step of the synthesis transforms p-acetamidobenzenesulfonyl chloride into p-acetamidobenzenesulfonamide by reaction with ammonia.
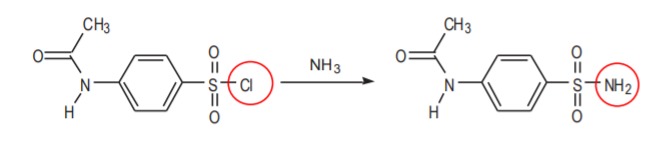
LIkewise, on p. 395 at the top, the textbook recommends that the organic sulfonyl chloride be handled expeditiously after pouring it into ice to avoid the slow decomposition that results from contact with water.
This makes it apparent why aniline cannot be used as the starting material in the synthesis. The amino group of aniline is a strong electrophile. This would cause undesirable side reactions in the first and second steps because the molecule would tend to react as a base or as a nucleophile to form undesirable products.
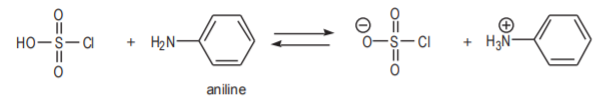
Aniline is a strong base. It can react with chlorosulfonic acid in a proton exchange in the first step.
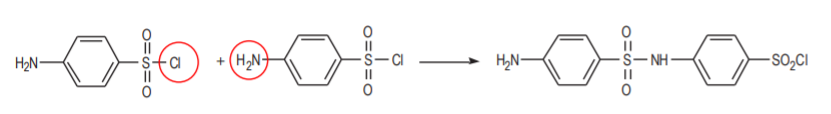
Even if this product could be prepared in the first step, it would react with itself in the second one as a nucleophile.


