10.2: Protecting Groups
- Page ID
- 220420
An abbreviated representation of this synthesis is shown below. Both the starting material and the product contain a benzene ring as the basic carbon skeleton. There are nitrogen-containing groups attached to both molecules. In the starting material this group is an amide. In the product it is an amine.
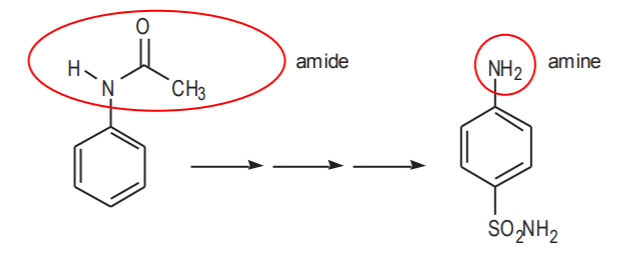
Notice that the amide group is carried all the way through the synthesis. It is not modified into amine until the very end, when the final step is reached. The question then is, why not start the synthesis with an amine group in the first place? The amide group can be thought of as a protected amine. Amines are very reactive, whereas amides are not. The amide group is preferred because there is less probability of side reactions through the synthesis with a less reactive group than with a more reactive one. The CH3CO portion of the amide (called acetyl group) can be thought of as a protective group for the amine that keeps it from undergoing side reactions. Protecting groups are widely used in organic synthesis for better control during the intermediate steps. They are typically removed at the end of the synthesis when they are no longer needed.


