13.6: Some Important Experimental Notes
- Page ID
- 213770
1. Do not use boiling stones to boil a system containing strong acids. The acid will destroy the stones. Always use your magnetic spin vane to stir.
2. ALKENES HAVE A STRONG, NOXIOUS, RANCID ODOR. STAY IN THE HOOD AT ALL TIMES! If you must handle the product from this reaction outside the hood, always use a capped vial or closed container.
3. Do not rinse any items containing alkene or reaction products in the regular troughs or sinks. Rinse your vials with acetone in the hood into the sink or into the organic waste container, then rinse outside the hood if more cleaning is needed.
4. If you experience nausea or dizziness from smelling alkene vapors, step outside the lab and get some fresh air. You should feel better after a while. If you don’t feel better, advise your instructor immediately.
5. Remember to use an O-ring and an aluminum foil shield during the distillation step.
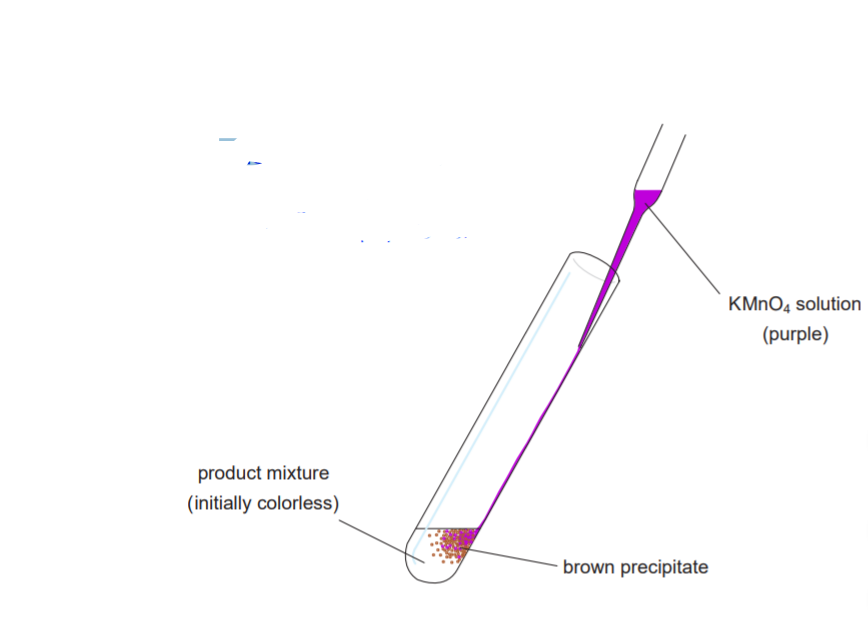
6. The book says to do two unsaturation tests (p. 211): one with bromine and one with KMnO4. DO NOT DO THE BROMINE TEST. DO ONLY THE KMnO4 TEST.
7. Ignore the unsaturation test description paragraph on p. 216 and refer to the picture on the next page instead. No 1,2- dimethoxyethane is needed, nor will it be available in the lab.
8. DO NOT DETERMINE THE BOILING POINT OF THE ALKENE, as instructed on p. 214 of the textbook. The smell in the lab will become unbearable. Take the IR spectrum instead, applying your sample on the IR card and quickly taking it to the instrument room for spectral recording.
9. Please clean up all sand spills from the distillation step. Leave the lab as you would like to find it.
UNSATURATION TEST
Alkenes change the color of potassium permanganate from purple to brown.