6: Steam Distillation of Methyl Salicylate, Hydrolysis of an Ester (Experiment)
- Page ID
- 61316
Methyl Salicylate (Oil of Wintergreen)
You will be steam distilling methyl salicylate. Carefully weigh out 2 g of the crude methyl salicylate (student prep) to the nearest 0.1 gram in a 50 or 100 mL rbf. Add 18 mL of water, several boiling stones, and setup your glassware for a simple distillation (your instructor will demonstrate) using a heating mantle. At or near 100oC, the mixture of water and MS will distill. Collect the distillate until it is clear or until most of the water has distilled.
To recover the methyl salicylate, carefully extract the oily distillate with 15 mL of methylene chloride two times. Combine the extracts and dry over anhydrous magnesium sulphate (1/4 tsp). Filter or decant into a tared 100 mL r.b. flask and concentrate on the roto-evaporator (you will use this flask to hydrolyze your ester). Weigh the residual product (to the nearest 0.1 g) and record this as recovered methyl salicylate and percent recovered methyl salicylate. This is the amount of MS that you use to calculate the amount of base to use for the hydrolysis and to calculate the theoretical yield of salicylic acid from recovered MS.
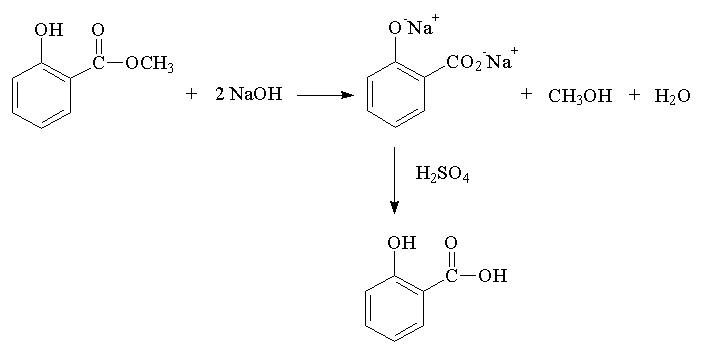
Hydrolyzing an ester. You will hydrolyze the recovered methyl salicylate in the 100 mL rbf using aqueous base. Calculate the amounts needed once you have weighed your recovered methyl salicylate. You will use 7 molar equivalents of base to each molar equivalent of methyl salicylate. One molar equivalent is needed to neutralize the acidic phenolic hydrogen, another will neutralize the newly formed carboxylic acid. The rest of the base is excess and must be neutralized with acid when the reaction is over. Sodium hydroxide is 40 mg/mmol compared to 152 mg/mmol for methyl salicylate. The sodium hydroxide is dissolved in sufficient water to make a 5M solution (eg, 5 mmol/mL or 200 mg would be dissolved in 1 mL of water).
Sample calculation: For 1.52 g of recovered MS which is 10 mmol of MS, you would use 70 mmol of base or 2.8 g of sodium hydroxide which you would dissolve in sufficient water to make 14 mL of solution or use 14 mL of a 5M NaOH solution.
Add the aqueous sodium hydroxide to the methyl salicylate in the 100 mL rbf. A white precipitate may form but it will dissolve upon heating. Add a boiling stone, a slightly greased reflux condenser (to prevent the standard tapers from "freezing" together due to the base attacking the glass), and reflux for 30 minutes using a heating mantle. When the solution has cooled, transfer it to a 100 mL beaker. The disodium salt of salicylic acid is in the basic solution. Rinse the rbf with only a few mL of water and transfer this wash to the same beaker.
To neutralize the basic solution and to acidify the disodium salt of the product, 1M sulfuric acid is used. Remember that 1M sulfuric acid is 2N sulfuric acid.
Calculate how much 2N sulfuric acid is needed to neutralize the amount of NaOH you actually used. You will need a slight excess so that the solution is definitely acidic in order to precipitate the product, salicylic acid. Check the acidity with blue litmus paper, when it turns pink, add an additional 5-10% of the sulfuric acid to precipitate the SA.
Sample calculation: Based on the example above where we used 70 mmol of base, which was 70 mequivalents of base, you would need 70 mequivalents of acid. 2N sulfuric acid is 2 mequiv/mL so you would need 35 mL, plus another 2-3 mL.
Cool the solution to 0oC. Filter the crude salicylic acid using a Buchner funnel. Check the filtrate with blue litmus paper to be certain it is acidic. If not, add additional 2N sulfuric acid to see if additional SA precipitates. The crude SA will contain water even after it is air dried for several minutes so weighing the crude product provides only an approximate, maximum yield.
Recrystallize the crude SA from very hot water (approx. 20 mL/g) using an Erlenmeyer flask. Use a minimum amount of boiling water by adding it slowly and observing how much compound goes into solution. Use a boiling stone to provide ebullation. Re-read the section on crystallization (Manual, p. 11) if this is unfamiliar. Keep the solution warm either on a steam bath or a hot plate kept at a temperature of approx. 100oC. It may be necessary for you to gravity filter this solution. If so, this is one of the most difficult parts of the experiment since the solution and the glassware must be kept warm during the filtration otherwise the SA starts to precipitate too early. If necessary, gravity filter the hot solution using a piece of fluted filter paper in your powder funnel (ask for a demonstration). Keep all solutions warm or else the SA will precipitate in the funnel rather than passing through into the receiver. If this occurs, it will be necessary to use hot water (or heated filtrate) to redissolve the solid. Speak to your instructor if this occurs.
The hot solution is cooled in an ice-water bath until precipitation appears to have ceased. The recrystallized salicylic acid is vacuum filtered, air dried for a few minutes, and then left in an open container to dry until the next lab period. At that time, you can weigh your dry sample and calculate your % yield. Record the melting point (lit mp 159-160o).
Questions
- What happened to the methanol produced in the reaction?
- What is the solid that forms when the methyl salicylate is added to aqueous base?
- If you calculated that you needed 10 mL of 2N sulfuric acid but you discover that the only acid available is 5M hydrochloric acid, how many mL would you use?
- Why is it necessary to have so much excess base?
- From PV=nRT we can obtain the ratio Pw/PMS = nw /nMS when V and T are constant.
At close to 100oC, the vapor pressure of methyl salicylate (MS) is 12 mm, that of water (W) is almost 760 mm, the MW are 152 and 18 g/mol, respectively. Using this data and the equation, what is the ratio of gw/gMS that you expect to obtain in this distillation?
Contributors and Attributions
James Chickos, David Garin, and Valerian D'Souza. University of Missouri–St. Louis; Chemistry)

