2.2: Day 2 Procedures - Gass Chromatography
- Page ID
- 211992
Day 2: Part B Gas Chromatography
Part B. Gas Chromatography: Each team should run the following three GC’s:
(a) the original oil
(b) fraction 1(limonene)
(c) fraction 3 (carvone)
Read the general references on Gas Chromatography in Techniques in Organic Chemistry (MHSM). Detailed instructions for use of the Hewlett-Packard 5890-II gas chromatographs are provided in the Appendix at the end of this experiment.
Save a small portion (5 drops) of your original essential oil sample and of the distilled limonene and carvone fractions for Gas Chromatography and Refractive Indexes measurements. The composition of these samples will be analyzed to determine the effectiveness of the separation. Follow the procedures in the appendix carefully for preparation of the gas chromatograph samples.
The two biggest problems with sample preparation are:
- Not carrying out the double dilution (use disposable test tubes) for the GC analysis (thus the sample is too concentrated to get clean separation with minimal background noise and may overload the column)
- Not placing the barcode label in exactly the right spot for the instrument to read the label (thus causing the instrument to shutdown). Be sure to record barcode numbers as well as vial positions in the GC line-up.
Day 2: Part C. Synthesis of Semicarbazone

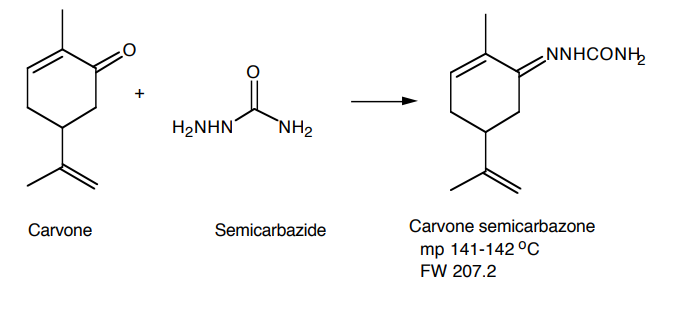
There are two diastereoisomers for the semicarbazone of (-)-carvone and (+)- carvone. They result from the restricted rotation about >C=N- bond. The α -isomer of (-)

or (+)-carvone melts at 162-3 °C, the b-isomer at 141-2 °C. Students will obtain either α -or the β -isomer depending on the actual conditions used. Because the limonene does not have a carbonyl group, the small amount of it, which remains in the carvone fraction, will not form a semicarbazone derivative. The limonene will remain in solution and be washed away during filtration.
In a large test tube, dissolve 0.5 g (4.5 mmole) of semicarbazide hydrochloride and 0.5 g of anhydrous sodium acetate (or 0.8 g of sodium acetate trihydrate) in 4 mL of distilled water and 7 mL of ethanol. Add 0.5 mL (0.48 g, 3.2 mmole,) of your carvone fraction. Stopper and shake your tube vigorously. Remove the stopper; place a boiling chip in the test tube and place in a beaker of warm water (ca. 80 - 90 °C) for 30 minutes. Careful! Contents of the test tube will boil. Beware of splashing!
There are three ways by which you can isolate the crystals of your semicarbazone derivative:
- You can add 3 mL of water and then set the reaction mixture aside to cool slowly at room temperature. Under these conditions, crystallization may take 45 minutes or longer. Slow crystal formation gives quite pure crystals that probably need not be recrystallized for the optical activity studies.
- Alternatively, after allowing the reaction mixture to cool for ten minutes or so, you can add three grams of ice or cold water and cool the mixture to 0-5 °C with waterice bath. If no crystals form, scratch the sides of the flask with a glass rod in order to induce crystallization.
- Set up a crystallization apparatus using one of the techniques discussed in lecture.
Collect the crystals by suction filtration using your Hirsch funnel and wash them with a few milliliters of cold water (do not forget to remove the boiling chips, hint: remove boiling chips by decanting the solution before crystallization begins).
Dry the semicarbazone in your desk until the next laboratory session.

