1.1: Introduction and Backdround
- Page ID
- 211989
Introduction
In this experiment2 you will be working with oils prepared from caraway seeds and spearmint leaves. Each oil has a distinct and characteristic odor, yet carvone is the major component in both oils! It is amazing that the difference in odor is attributable solely to a difference in chirality of the carvone in the two oils. Due to chirality of odor receptors in the nose the R-carvone and S-carvone fit into different receptor sites, hence different odor. Can you distinguish between the odors? 8-10% of the population cannot.3 Some physical data4 are presented below.

All the physical properties should be identical except for the optical rotations of the two isomers (enantiomers), which are of opposite sign. Thus, for both (+)- and (-)- carvone, the infrared, nuclear magnetic resonance spectra, the gas chromatographic retention times, the refractive indexes, and the boiling points should be identical. Hence, the only difference in properties one should observe for the two carvones are the odors and the signs of rotation in a polarimeter. However, some of the physical properties presented above are not identical because of trace impurities.
The * in the formulas above denotes a chiral carbon center. Chiral or asymmetric compounds in nature exist only in living tissue or in matter that was once part of living tissue. Chirality plays a major role in the mechanisms of biological recognition. Yet it is a mystery why caraway plants, Carum carvi, produce S-(+)-carvone and spearmint plants produce its mirror image (R)-(-)-carvone. Other plants such as gingergrass produce racemic carvone. Nature goes one step further; some botanically indistinguishable plants grown in different countries can carry out complete metabolic sequences of mirror-image reactions. Presumably, the enzymes that catalyze the reactions also have a mirror-image relationship. Another example of chiral recognition5 is found in the effect these two carvone isomers have on rates of reaction. The toxicity of S-(+)-carvone in rats is 400 times greater than that of (R)-(-)-carvone.
Essential oils are extracts from fragrant plants. They are used extensively in the perfume and flavoring industry. Most components of essential oils are terpenes that contain multiples of a five carbon structural unit, the isoprene unit (Fig. 1).
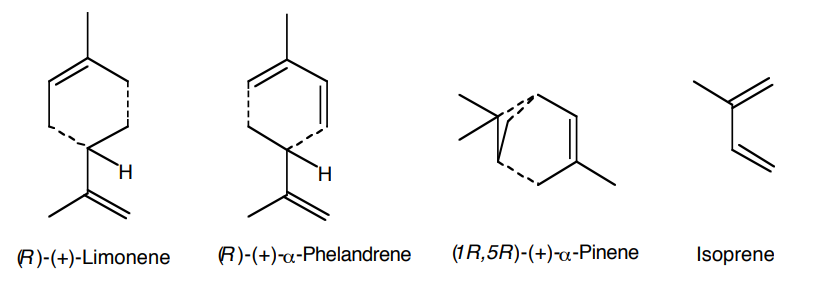
Figure 1. Representative monoterpenes. Isoprene units are shown to indicate the common structural features.
In addition to monoterpenes, compounds derived from two isoprene units, essential oils contain less volatile compounds derived from three and four isoprene units. These higher boiling components will be removed by vacuum distillation of the provided sample to permit facile gas chromatographic separation.
Overview of the Experiment
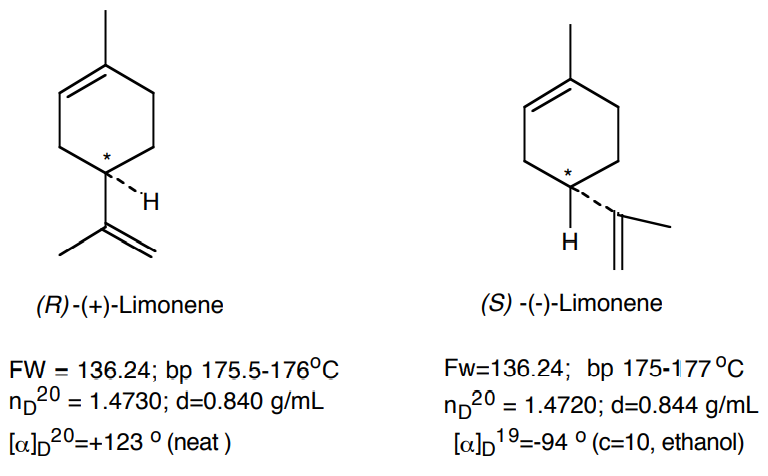
- You will be given a sample of either caraway oil or spearmint oil. The major component of these oils is carvone. You will separate the carvone from the higher-boiling and lower-boiling impurities (such as limonene), via vacuum distillation.
- You will use gas chromatography and refractometry to check the purity of your distillate and to estimate the relative concentrations of limonene and carvone in the oil.
- You will convert the carvone to its semicarbazone for use in a polarimetric analysis.
- You will obtain infrared spectra of the carvone and limonene fractions and interpret the results.
- You will also characterize the semicarbazone by melting point determination.
- Visit the X-Ray Crystallography laboratory where spectra of selected crystals will be determined.
Background for Experimental Procedure
General References
- Distillation MHS, Chapter 12, pp 173-205
- Vacuum Pumps TM(I) Sec. 11C.
- Gas Chromatography MHS, Chapter 20, pp 291-308
- Polarimetry MHS, Chapter 17, pp 240-251
- Refractometry MHS, Chapter 13, pp 206-211
- Infrared Spectroscopy MHS, Chapter 21, pp 311-344
Videos: Digital Laboratory Techniques Manual
#7. Filtration
#11. Balances
#12. Melting Points
#15,16. Distillation I, II
Distillation
The difference between the boiling points of carvone (230 °C @ 760 torr) and limonene (177 °C @ 760 Torr) is sufficient to permit separation of the two compounds by distillation. However, carvone thermally decomposes at higher temperatures; therefore, a vacuum distillation is necessary.
Two problems are encountered in a vacuum distillation. The volume of vapor formed from a given amount of liquid is pressure dependent; i.e., the volume of vapor formed from one drop of liquid will be about 30 times as great at 25 torr as it was at 760 torr. As a result, serious bumping may occur. Boiling chips generally do not help much at the reduced pressures. Some of the bumping can be overcome with the use of a magnetic stir bar. The second problem is also related to the larger volume of vapor at lower pressure. The velocity of the vapor entering the column is greatly increased. This creates a greater pressure in the column than is registered on the manometer. Maintaining a slow, steady rate of distillation can minimize this difference in pressure.
Gas Chromatography and Refractometry
In Gas Liquid Chromatography a mixture of vapors is carried in a stream of helium (carrier gas) through a column. The vaporized sample components move through the column that is lined with a liquid stationary phase. The different components in the sample are retained on the stationary phase for different characteristic relative times. Each component ultimately reaches the Flame Ionization Detector, the most commonly used detector in GC (Air + Hydrogen gas, ratio 10:1). They are detected by their ability to form ions when they are burned in the H2 / air mixture. The area under a peak in a gas chromatogram is proportional to the amount of that substance in the sample.
Among the factors that influence the separation of compounds by gas chromatography are selection of liquid phase, column temperature, and flow rate of carrier gas. Two common liquid (stationary) phases are silicone oil, which separates components on the basis of boiling point, and carbowax (polyethyleneglycols), which separates components on the basis of polarity. The effect of increased column temperature is to decrease the retention time of a component. As a rough approximation, a 10-15 °C decrease in column temperature corresponds to a doubling in the retention time. For most samples, the lower the column operating temperature, the higher the partition coefficient in the stationary phase, and hence, the better the separation. Too low a column temperature can lead to broad, asymmetric peak shapes. The criterion for resolution of the sample is simply achieving baseline separation of the components. Varying the column temperature and selecting the appropriate liquid phase will achieve resolution of the sample into its components. Identification of retention time can be accurately obtained using a pentane peak as a standard. There will always be enough pentane in the syringe to leave a small peak on the chromatogram. The retention time of the other peaks can be calculated using the pentane peak. The relative amounts of carvone and limonene in each fraction and the original oil may be calculated by using the area under the appropriate peaks.
By measuring the refractive index of the original oil, limonene and carvone fractions, you can estimate the purity of the respective fractions and the composition of the original oil. Assuming that the actual refractive index, n, measured for the twocomponent mixture (limonene and carvone) is linear in the molar fraction, x, of any of the components, then one can write:
\[ \rm n = (1-x_{carvone}) * n_{limonene} + x_{carvone} * n_{carvone}\]
Plug in your data and determine the value of xcarvone for the limonene and carvone distillation fractions and the oil itself. Compare these results with those obtained by GC.
Footnotes
1 The experiment includes contributions from past instructors, course textbooks, and others affiliated with course 5.310 updated by John Dolhun May 2017.
2 Adapted from: Pavia, D. L.; Lampman, G. M.; Kriz, G. S.; Engel, R. G. “Introduction to Organic Laboratory Techniques”; Saunders: Philadelphia, PA, 1990, pp. 96-107.
3 ibid. p.103.
4 Physical data is taken from Aldrich Chemical Catalog 1998-1999
5 The phenomenon in which a chiral receptor interacts differently with each of the enantiomers of a chiral compound is called chiral recognition.

