2.2: Day 2 Procedure - Purification
- Page ID
- 211978
Day 2 - Purification of Ferrocene and Acetylation of Ferrocene
Ferrocene is a crystalline, diamagnetic material that is extremely stable to air, moisture and light. It is moderately to extremely soluble in practically all-nonpolar or weakly polar solvents. It may be purified by sublimation.
Weigh and take a mp of the dried crude ferrocene. [NOTE: All melting points must be taken in melting point tubes sealed off with parafilm. Ferrocene sublimes below its melting point and would be lost from an unsealed tube.]
Sublimation may be conducted in a 100 x 15 mm culture dish as shown in figure 2 or with the apparatus demonstrated by your TA. Transfer ferrocene to the "bottom" of the culture dish to cover the center of the dish to a thickness of about 5 mm. Spread the sample out don’t pile it up. Cover with the larger half of the culture dish and place it on a variable temperature hot plate. Slowly raise the temperature setting the hot plate to position 2 until the ferrocene sublimes to the upper half of the dish. The sublimation will proceed slowly. Cooling the top culture dish by placing a beaker filled with ice water on top of it will facilitate the sublimation. (WARNING: Slide the beaker off the top of the culture dish. Lifting it may lift the upper culture dish off and cause it to fall or disturb the sublimed ferrocene resulting in a loss of ferrocene.) Allow the dish to cool completely before removing from the hotplate, then, recover the sublimed ferrocene. This procedure may be repeated several times until all the ferrocene is purified.9 Do not heat over 100 °C.
Determine the melting point of each batch of ferrocene sublimed. Place the final product in a weighed vial, determine the yield and report this along with the melting point. Calculate and report your actual percent yield. The melting point should equal or exceed 171 °C (lit. 173-174 °C).
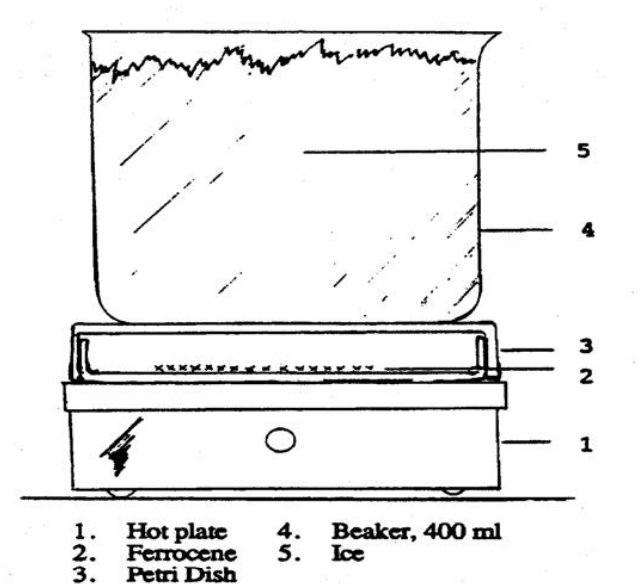
Figure 2: Apparatus for the Sublimation of Ferrocene
Ferrocene will be acetylated by the action of phosphoric acid* and acetic anhydride*. The number of products in the reaction mixtures will be determined by thinlayer chromatography and the products isolated by column chromatography.
Acetylation Reaction
Place a 3x10 mm stir bar in a 10 mL round bottom flask (RBF). Prepare a 65˚C water bath (use the red liquid thermometer and small crystallizing dish) and preheat the flask in the dish (temperature is critical higher temperatures may destroy your products). Then add, in the following order, 93±3 mg (0.5 mmol) of ferrocene, 0.5 mL of acetic anhydride, and 0.1 mL of 85% phosphoric acid. (WARNING: changing the order of addition will likely result in a brown goo resulting from the decomposition of the ferrocene, careful measurement of all quantities involved is also crucial to the success of this reaction.) Cap the RBF with a red 14x20 septum, insert an empty syringe needle, and warm it in the water bath while agitating the mixture to dissolve the ferrocene. Heat the mixture for 30 minutes.
Cool the 10 mL, round bottom flask (RBF) thoroughly in an ice bath. Carefully add to the solution 0.5 mL of ice water. Mix thoroughly. Add dropwise 3 M aqueous sodium hydroxide solution until the mixture is neutral (50+ drops, test with indicator paper around 40 drops and then each 5 drops to avoid excess base).
Collect the product on a Hirsch funnel, wash with 4x1.0 mL of water, and press it as dry as possible between sheets of filter paper. Dry the crystals until the next lab session.
Footnotes
9 Acetone* may generally be used to clean glassware during this synthesis. A concentrated solution of KOH in water may be used to clean the petri dishes after sublimation.

