8.1: Classifying Acids and Bases
- Page ID
- 52175
Skills to Develop
- List the properties of acids.
- List the properties of bases.
- Define an Arrhenius acid and list some substances that qualify as acids under this definition.
- Define an Arrhenius base and list some substances that qualify as bases under this definition
Introduction
We may not realize how much acids and bases affect our lives. Have you every though of drinking a can of soda pop and actually drinking acid? Have you looked at bottles of household cleaners and noticed what the main ingredients were? Have you ever heard a shampoo commercial and heard them say that the shampoo was "pH balanced" and wondered what this means and why it is so important for hair? Thanks to the beginning work of scientists in the latter part of the 19\(^\text{th}\) century, we started to learn about acids and bases; our study continued and is constantly growing. Let's begin our study of this wonderful branch of chemistry.
Properties of Acids
Acids are a special group of compounds with a set of common properties. This helps to distinguish them from other compounds. Thus, if 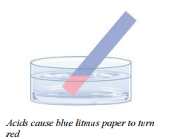 you had a number of compounds and you were wondering whether these were acids or otherwise, you could identify them by their properties. But what exactly are the properties? Think about the last time you tasted lemons. Did they taste sour, sweet, or bitter? Lemons taste sour.This is a property of acids. Another property of acids is that they turn blue litmus paper red. Litmus paper is an indicator, which is a substance that changes color depending on how acidic or basic something is. If blue litmus paper turns red when it is dipped into a solution, then the solution is an acid. Another property of acids that many people are familiar with is their ability to cause burns to skin. This is why it is a bad idea to play with battery acid or other acids.
you had a number of compounds and you were wondering whether these were acids or otherwise, you could identify them by their properties. But what exactly are the properties? Think about the last time you tasted lemons. Did they taste sour, sweet, or bitter? Lemons taste sour.This is a property of acids. Another property of acids is that they turn blue litmus paper red. Litmus paper is an indicator, which is a substance that changes color depending on how acidic or basic something is. If blue litmus paper turns red when it is dipped into a solution, then the solution is an acid. Another property of acids that many people are familiar with is their ability to cause burns to skin. This is why it is a bad idea to play with battery acid or other acids.
Acids react with many metals to produce hydrogen gas. For some examples, look at the reactions below:
\[\ce{Zn} \left( s \right) + 2 \ce{HCl} \left( aq \right) \rightarrow \ce{ZnCl_2} \left( aq \right) + \ce{H_2} \left( g \right)\]
\[\ce{Mg} \left( s \right) + 2 \ce{HCl} \left( aq \right) \rightarrow \ce{MgCl_2} \left( aq \right) + \ce{H_2} \left( g \right)\]
What do you notice that is the same for both equations? In each case, the reactants are a metal (\(\ce{Zn}\) or \(\ce{Mg}\)) and an acid (\(\ce{HCl}\)). They all produce hydrogen gas, \(\ce{H_2}\). This is another property of acids. Acids react with most metals to produce hydrogen gas.
Think about the last time you took an aspirin or a vitamin C tablet. Aspirin is acetylsalicylic acid while vitamin C is ascorbic acid; both are acids that can produce \(\ce{H^+}\) ions when dissolved in water. Acetic acid (\(\ce{HC_2H_3O_2}\)) is a compound of vinegar, hydrochloric acid (\(\ce{HCl}\)) is stomach acid, phosphoric acid (\(\ce{H_3PO_4}\)) is commonly found in dark soda pop, sulfuric acid (\(\ce{H_2SO_4}\)) is used in car batteries, and formic acid (\(\ce{HCO_2H}\)) is what causes the sting in ant bites. For all of these acids, the chemical formula of an acid begins with one or more hydrogen atoms. Acids dissolve in water to make \(\ce{H^+}\) ions. Because they make ions (charged particles) when they are dissolved, acids will also conduct electricity when they are dissolved in water.
We interact with acids on a daily basis so some knowledge of their properties and interactions is essential. Acids are present in our everyday lives.
Properties of Bases
There is one common base that some may have had the opportunity to taste: milk of magnesia, which is a slightly soluble solution of magnesium hydroxide. This substance is used for acid digestion. Flavorings have been added to improve taste, otherwise it would have 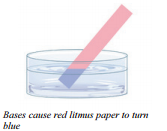 a bitter taste when you drink it. Other common bases include substances like Windex, Drano, oven cleaner, soaps, and many other cleaning products. Please note: do not taste any of these substances. A bitter taste is one property you will have to take for granted. Bases also tend to have a slippery feel. This matches what you have experienced with soaps and detergents.
a bitter taste when you drink it. Other common bases include substances like Windex, Drano, oven cleaner, soaps, and many other cleaning products. Please note: do not taste any of these substances. A bitter taste is one property you will have to take for granted. Bases also tend to have a slippery feel. This matches what you have experienced with soaps and detergents.
As with acids, bases have properties that allow us to distinguish them from other substances. We have learned that acids turn blue litmus paper red. Bases turn red litmus paper blue. Notice that the effect of the indicator is the opposite of that of acids.
Most acids have formulas that start with \(\ce{H}\). On the other hand, most of the bases we will be using in this course have formulas that end with \(\ce{-OH}\). These bases contain the polyatomic ion called hydroxide. When bases dissolve in water, they produce hydroxide (\(\ce{OH^-}\)) ions. Because they dissolve into charged particles, bases will also conduct electricity when they are dissolved.
Although many people have already heard of the danger of acids causing burns, many bases are equally dangerous and can also cause burns. It is important to be very careful and to follow correct safety procedures when dealing with both acids and bases.
Acids & Bases Defined
Although scientists have been able to classify acids and bases based on their properties for some time, it took a while to come up with a theory explaining why some substances were acidic and others were basic. Svante Arrhenius set the groundwork for our current understanding of acid-base theory. We will focus on his famous acid-base definitions. This was quite an accomplishment for a scientist in the late 19\(^\text{th}\) with very little technology, but with the combination of knowledge and intellect available at the time Arrhenius led the way to our understanding of how acids and bases differed, their properties, and their reactions. Keep in mind that Arrhenius came up with these theories in the late 1800's so his definitions came with some limitations. For now we will focus on his definitions.
Arrhenius Acids
Take a look at all of the following chemical equations. What do you notice about them? What is common for each of the equations below?
Hydrochloric acid: \(\ce{HCl} \left( aq \right) \rightarrow \ce{H^+} \left( aq \right) + \ce{Cl^-} \left( aq \right)\)
Nitric acid: \(\ce{HNO_3} \left( aq \right) \rightarrow \ce{H^+} \left( aq \right) + \ce{NO_3^-} \left( aq \right)\)
Perchloric acid: \(\ce{HClO_4} \left( aq \right) \rightarrow \ce{H^+} \left( aq \right) + \ce{ClO_4^-} \left( aq \right)\)
One of the distinguishable features about acids is the fact that acids produce \(\ce{H^+}\) ions in solution. If you notice in all of the above chemical equations, all of the compounds dissociated to produce \(\ce{H^+}\) ions. This is the one main, distinguishable characteristic of acids and the basis for the Arrhenius definition of acids. An Arrhenius acid is substance that produces \(\ce{H^+}\) ions in solution
Arrhenius Bases
In contrast, an Arrhenius base is a substance that releases \(\ce{OH^-}\) ions in solution. Many bases are ionic substances made up of a cation and the anion hydroxide, \(\ce{OH^-}\). The dissolving equation for the base sodium hydroxide, \(\ce{NaOH}\), is shown below:
\[\ce{NaOH} \left( s \right) \rightarrow \ce{Na^+} \left( aq \right) + \ce{OH^-} \left( aq \right)\]
Barium hydroxide produces a similar reaction when dissociating in water:
\[\ce{Ba(OH)_2} \left( s \right) \rightarrow \ce{Ba^{2+}} \left( aq \right) + 2 \ce{OH^-} \left( aq \right)\]
The production of \(\ce{OH^-}\) ions is the definition of bases according to Arrhenius.
Lesson Summary
- Acids turn blue litmus paper red, taste sour, and react with metals to produce hydrogen gases.
- Common acids include vinegar (\(\ce{HC_2H_3O_2}\)), phosphoric acid in soda pop (\(\ce{H_3PO_4}\)), and stomach acid (\(\ce{HCl}\)).
- Bases turn red litmus paper blue, have a bitter taste, and are slippery to the touch.
- Common bases include Drano (\(\ce{NaOH}\)), soaps and detergents, milk of magnesia (\(\ce{Mg(OH)_2}\)), and Windex (\(\ce{NH_4OH}\)).
- Arrhenius defined an acid as a substance that donates \(\ce{H^+}\) ions when dissociating in solution.
- An Arrhenius base is a substance that releases \(\ce{OH^-}\) ions in solution.
Vocabulary
- Arrhenius acid: A substance that produces \(\ce{H^+}\) ions in solution.
- Arrhenius base: A substance that produces \(\ce{OH^-}\) ions in solution.
Further Reading/Supplemental Links
- Strong & Weak Acids animation: http://www.mhhe.com/physsci/chemistr...1/acm2s1_1.htm
- Tutorial: Acids & Bases http://visionlearning.com/library/mo...p?mid=58&1=&c3=

