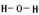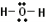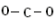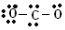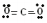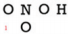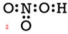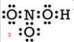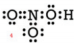4.6: Covalent Compounds & Lewis Structures
- Page ID
- 49847
Skills to Develop
- Explain what covalent bonds are.
- Explain why covalent bonds are formed.
- Draw Lewis structures for covalent compounds and polyatomic ions.
In ionic bonding, electrons leave metallic atoms and enter nonmetallic atoms. This complete transfer of electrons changes both of the atoms into ions. Often, however, two atoms combine in a way that no complete transfer of electrons occurs. Instead, electrons are held in overlapping orbitals of the two atoms, so that the atoms are sharing the electrons. The shared electrons occupy the valence orbitals of both atoms at the same time. The nuclei of both atoms are attracted to this shared pair of electrons and the atoms are held together by this attractive force. The attractive force produced by sharing electrons is called a covalent bond.
Covalent Bond Formation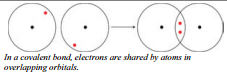
In covalent bonding, the atoms acquire a stable octet of electrons by sharing electrons. The covalent bonding process produces molecular substances as opposed to the lattice structures of ionic bonding. There are far more covalently bonded substances than ionic substances.
 The diatomic hydrogen molecule, \(\ce{H_2}\), is one of the many molecules that are covalently bonded. Each hydrogen atom as a \(1s\) electron cloud containing one electron. These \(1s\) electron clouds overlap and produce a common volume which the two electrons occupy.
The diatomic hydrogen molecule, \(\ce{H_2}\), is one of the many molecules that are covalently bonded. Each hydrogen atom as a \(1s\) electron cloud containing one electron. These \(1s\) electron clouds overlap and produce a common volume which the two electrons occupy.
Some Compounds Have Both Covalent and Ionic Bonds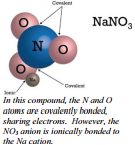
If you recall the introduction of polyatomic ions, you will remember that the bonds that hold the polyatomic ions together are covalent bonds. Once the polyatomic ion is constructed with covalent bonds, it reacts with other substances as an ion. The bond between a polyatomic ion and another ion will be ionic. An example of this type of situation is in the compound sodium nitrate. Sodium nitrate is composed of a sodium ion and a nitrate ion. The nitrate ion is held together by covalent bonds and the nitrate ion is attached to the sodium ion by an ionic bond.
Lewis Structures
The Lewis Structure of a molecule shows how the valence electrons are arranged among the atoms of the molecule. These representations are named after G. N. Lewis. The rules for writing Lewis structures are based on observations of thousands of molecules. From experiment, chemists have learned that when a stable compound forms, the atoms usually have a noble gas electron configuration or eight valence electrons. Hydrogen forms stable molecules when it shares two electrons (sometimes called the duet rule). Other atoms involved in covalent bonding typically obey the octet rule. (Note: Of course, there will be exceptions.)
To draw a Lewis structure:
1. Determine the number of valence electrons that will be drawn in the Lewis structure.
a. Use your periodic table to determine the number of valence electrons in each atom. Add these to get the total electrons in the structure.
b. If you are drawing the structure for a polyatomic ion, you must add or subtract any electrons gained or lost. If an ion has a negative charge, electrons were gained. If the ion has a positive charge, electrons were lost.
2. Draw a skeleton
a. Typically, the first element listed in the formula goes in the center, which the remaining atoms surrounding.
b. Draw bonds to each of the surrounding atoms. Each bond is two valence electrons.
3. Use the remaining electrons to give each atom an octet (except hydrogen which only gets a duet)
a. Place electrons left over after forming the bonds in the skeleton in unshared pairs around the atoms to give each an octet. *Remember, any bonds they have formed already count as two valence electrons each.
b. If you run out of electrons, and there are still atoms without an octet, move some of the electrons that are not being shared to form double, sometimes triple bonds.
| Example: Draw a Lewis structure for water, \(\ce{H_2O}\). |
|
Solution: 1) Add up all available valence electrons: each \(\ce{H}\) atom has 1, each oxygen atom has 6, so \(2 \left( 1 \right) + 6 = 8\) 2) Draw a skeleton. Although the first atom written typically goes in the middle, hydrogen can't, so \(\ce{O}\) gets the middle spot. We need to draw bonds connecting atoms in the skeleton. We get:
3) Use the remaining electrons to give each atom (except hydrogen) an octet. If we look at our skeleton, we drew two bonds, which uses 4 of our 8 available electrons. We are left with four more. Each \(\ce{H}\) atom already has two valence electrons and \(\ce{O}\) currently has 4 (each bond counts as two for each atom that it connects). We will give the remaining four electrons to \(\ce{O}\) in pairs. We get:
Check: Is the total number of valence electrons correct? Yes. Our final picture has 8 valence electrons. Does each atom have the appropriate duet or octet of electrons? Yes. |
| Example: Draw a Lewis structure for \(\ce{CO_2}\). |
|
Solution: 1) Add up all available valence electrons: \(1 \left( 4 \right) + 2 \left( 6 \right) = 16\) 2) Draw a skeleton. Carbon goes in the middle with the two oxygen atoms bonded to it:
3) Use the remaining electrons to give each atom (except hydrogen) an octet. In this case, we have already used up four electrons to draw the two bonds in the skeleton, leaving 12 left. This is not enough to give everybody an octet. Our picture may look something like this with 16 electrons:
We have used up the 16 electrons, but neither \(\ce{O}\) has an octet. The rules state that if you run out of electrons and still don't have octets, then you must use some of the unshared pairs of electrons as double or triple bonds instead. Move the electrons that are just on the carbon atom to share with the oxygen atom until everybody has an octet. We get:
Check: Is the total number of valence electrons correct? Yes. Our final structure has 16 valence electrons. Does each atom have the appropriate duet or octet of electrons? Yes. |
|
Example: Draw a Lewis structure for nitric acid, \(\ce{HNO_3}\). The skeleton is given below:
|
|
Solution: 1) Add up all available valence electrons: \(1 \left( 1 \right) + 1 \left( 5 \right) + 3 \left( 6 \right) = 24\) 2) Draw a skeleton. This was given to use, but we need to draw the bonds.
3) Use the remaining electrons to give each atom (except hydrogen) an octet. Each bond used up 2 electrons, so we have already used 8 electrons. If we use the remaining 16 electrons, we may get a picture such as:
But notice that the nitrogen atom still does not have an octet. We ran out of electrons so we must form a double bond. Use some of the electrons on an oxygen atom to share with the nitrogen. We get:
Check: Is the total number of valence electrons correct? Yes. Our final structure has 24 valence electrons. Does each atom have the appropriate duet or octet of electrons? Yes. |
Lesson Summary
- Covalent bonds are formed by electrons being shared between two atoms.
- Half-filled orbitals of two atoms are overlapped and the valence electrons shared by the atoms.
- Covalent bonds are formed between atoms with relatively high electron affinity.
Vocabulary
- Covalent bond: A type of bond in which electrons are shared by atoms.
Further Reading/Supplemental Links
- Tutorial bon bonding: http://visionlearning.org/library/module_viewer.php?mid=55&l=