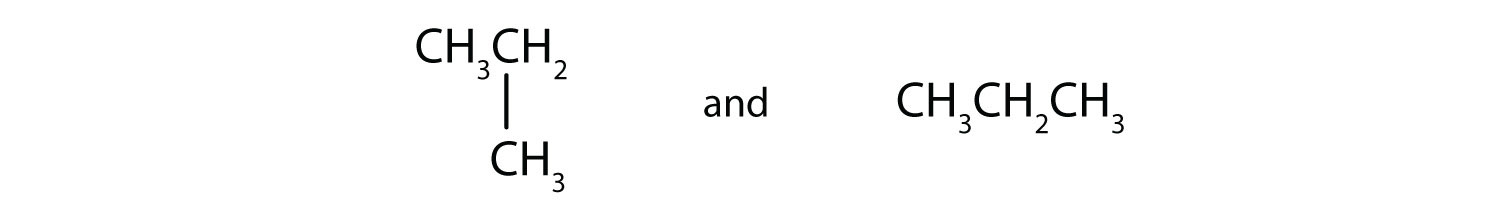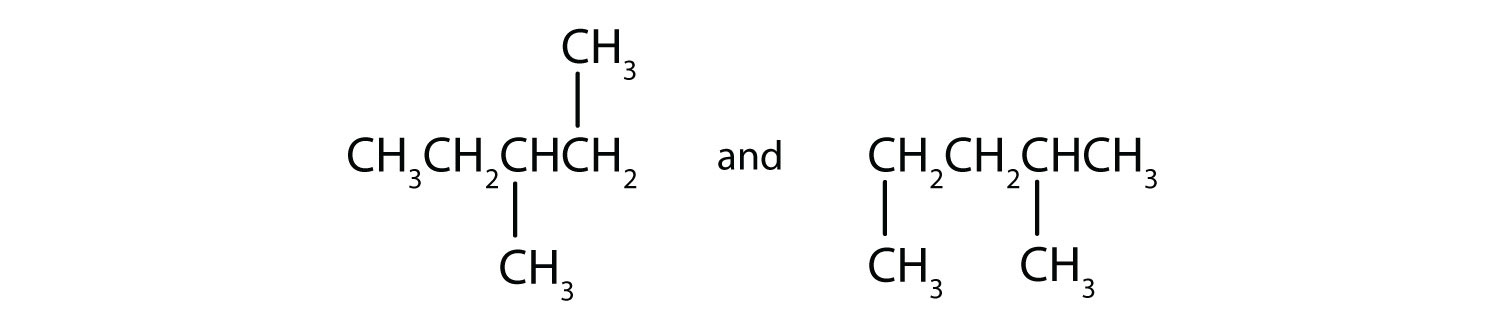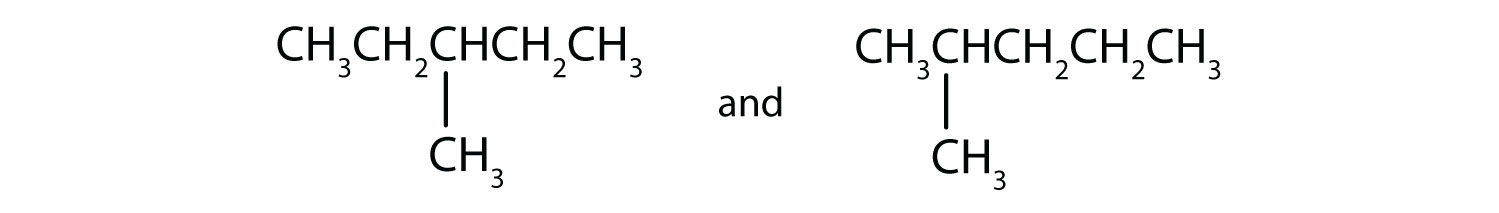8: Hydrocarbons Structure & Nomenclature: Questions
- Page ID
- 43922
Hydrocarbons
(01) Draw an example of each of the four types of hydrocarbons: alkane, alkene, alkyne, and aromatic.
(02) Answer True or False for the following statements.
- Organic molecules occur only in nature and cannot be artificially synthesized
- Medicines we consume are not derived from natural sources
- Benzene is an example of an aromatic compound
- Propane has one double bond
(03) Define the term saturation for "hydrocarbons", then determine if the following compounds are saturated or unsaturated.
- C2H6
- C3H6
- C6H6
- C4H10
- C5H12
(04) Describe the characteristics of hydrocarbons by comparing the following pairs and choosing the correct option.
- Hydrophobic or hydrophilic
- H-bond capable or incapable
- Water-soluble or lipid-soluble
- Covalent or ionic bonding
- Limited combinations or many combinations
(05) Two compounds of similar molar mass, but different chemical formulas have two very different boiling points. One compound is revealed to be a hydrocarbon. What must the difference be with the other compound for there to be such a difference in boiling point?
Saturated Hydrocarbons
(06) Answer the following true and false statements accordingly.
- Double and triple bonds are capable of rotation.
- Structural isomers have the same chemical formula, but different bonding patterns.
- A conformer is a completely different compound than the original.
- Single bonds can only partially rotate, up to 180 degrees.
- Only one carbon in a hydrocarbon is allowed to rotate.
(07) Sketch the two isomers of C4H10 and give the IUPAC name of each.
(08) What is the molecular geometry of an alkyne carbon? Of an alkene carbon? Of an alkane carbon? Which one of these is possible to be considered ‘saturated’?
(09) Examine the following pairs of molecules. Determine whether they are identical, completely different, or merely conformers of one another.
a.

b.

c.

d.

e.
Writing Structures
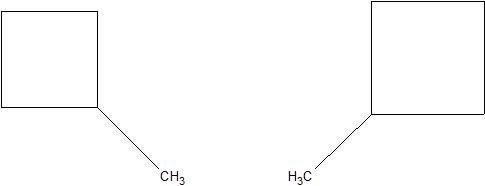
(10) Translate the following structures into their condensed formulas.
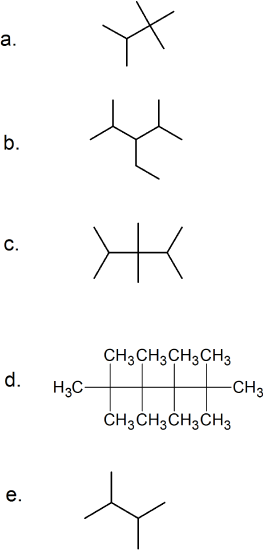
(11) Translate these skeletal structures into condensed formulas and provide the IUPAC name of each.
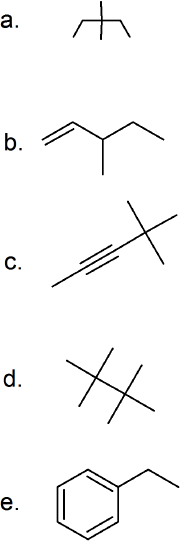
(12) Give the IUPAC name for each of the following compounds.
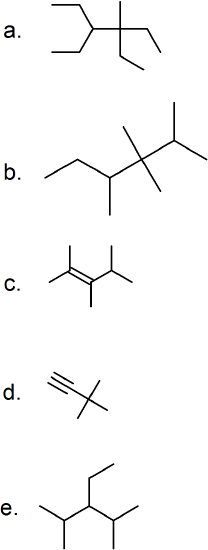
(13) Translate these expanded structures into their skeletal equivalents.
a.
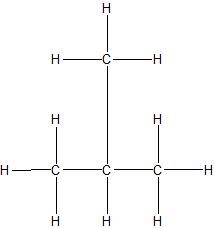
b.
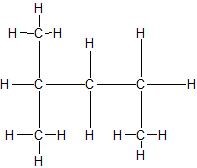
c.
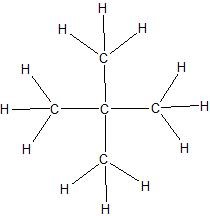
d.
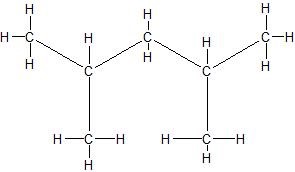
e.
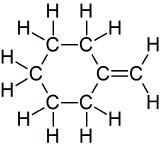
(14) Expand the given condensed structures into skeletal line structures.
a. 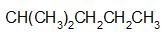
b. CH3(CH2)14CH=CH2
c. CH3CH2CH(CH3)CH(CH3)CH3
d.
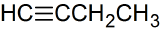
e. CH3C(CH3)2CH2CH=CHCH2CH3
(15) Is a cycloalkane saturated or unsaturated? Sketch a five-membered ring and give the bond-line structure, then compare it to a straight-chain hydrocarbon with five carbons to confirm whether there are more hydrogens in the ring or straight chain.
Unsaturated Hydrocarbons: Alkenes and Alkynes
(16) Classify each of the following unsaturated hydrocarbons either simple alkenes/alkynes, dienes/diynes, polyene/polynes, or aromatics.
a. 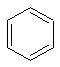
b. 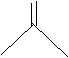
c. 
d. 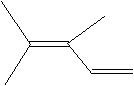
e. 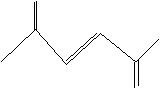
Naming Hydrocarbons
(17) Using your knowledge of alkane, alkene and alkyne nomenclature, name the following straight-chain molecules.
a. 
b. 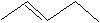
c. 
d. 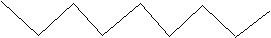
e. 
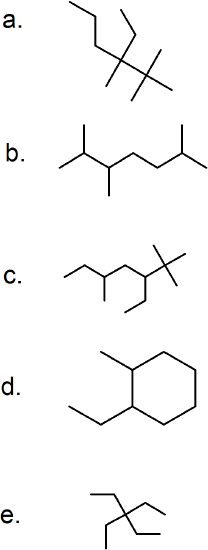
(18) Name each of the following branched alkanes and determine their molecular formulas.
(19) Convert these IUPAC names into their skeletal structures.
- trans-2,5-dimethyl-3-heptene
- 3-ethyl-5-propyloctane
- cis-2-methyl-1-ethylcyclopentane
- 4-ethyl-1-hexyne
- trans-4,5,6,7-tetramethyl-2-nonene
(20) Give the IUPAC names of the following skeletal structures.
a. 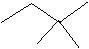
b. 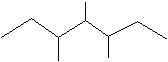
c. 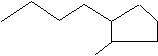
d. 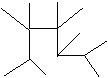
e. 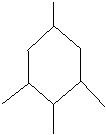
(21) Sketch the skeletal-line structures and chemical formulas for the following pairs of compounds to observe the differences and how even slight changes mean that compounds are structured entirely differently.
- Propane, Cyclopropane
- Ethyne, Ethene
- Cyclohexane, Benzene
- Octane, 1-Octene
- 1-pentene, cis-2-pentene
(22) Give the IUPAC name for the following alkenes and alkynes.
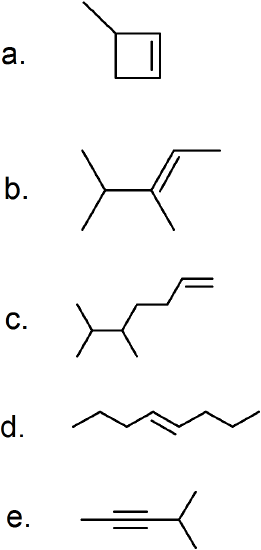
(23) Convert these IUPAC formulas into skeletal structures.
- Cyclooctane
- trans-1,3-dimethylcyclohexane
- 3,6-dimethyldecane
- cis-4-octene
- 2-hexyne
(24) For the following alkenes and alkynes, translate their IUPAC names into skeletal structures.
- cis-3-hexene
- 3-heptyne
- trans-2-butene
- 2-methyl-1-propene
- trans-3-ethyl-4-nonene
(25) Examine the provided alkynes and determine their IUPAC names.
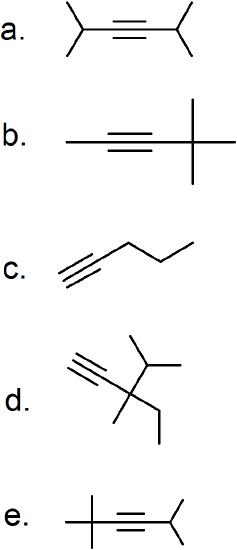
Aromatic Hydrocarbons
(26) Draw the bond-line structures and determine the chemical formulas for hexane, 1-hexene, cyclohexane, and cyclohexene. Write the mathematical formula for the ratio of carbon to hydrogen for each compound to determine the pattern.
(27) Like cycloalkanes, benzene can have multiple substituent groups. Sketch the skeletal structure for 3,4-dipropyl-2,5-methyl-1-ethylbenzene.
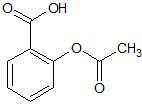
(28) Acetylsalicylic acid, more commonly known as aspirin, is a multisubstituted benzene compound. Is the substitution a 1,2-, a 1,3-, or a 1,4-? Once you’ve determined the correct combination, sketch what the molecule would look like for each of the other two possible substitutions.
(29) Two B-vitamins are shown below. Examine their structures in order to answer the related questions.
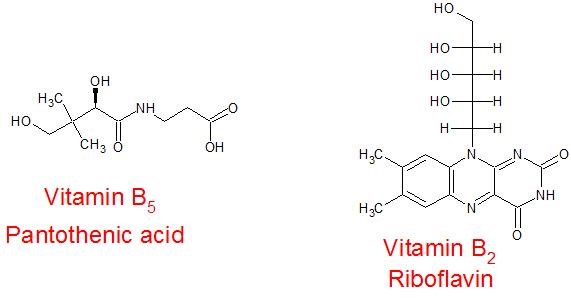
- Do one or both contain aromatic rings? Which one(s)?
- What is the longest straight carbon chain in either vitamin?
- Write the chemical formula for each vitamin.
- Would you consider these vitamins to be water-soluble or lipid-soluble? What characteristics or features support your answer?
- Does your answer to the previous question agree with the following information? Vitamins B5 and B2 are known to be more easily expelled in urine and so they need to be more frequently consumed in the diet or through supplements, unlike with vitamins A, D, E and K.
(30) Take a look at the two multi-substituted benzene compounds below. Trinitrotoluene (TNT) has its NO2 groups on carbons 2, 4, and 6. Which of the two fits this numerical scheme? Hint: Toluene is a special name for benzene whose primary functional group is a methyl group substituted in place of one of its hydrogens.
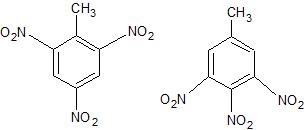
A B
Additional Exercises
(31) You find an unlabeled jar containing a solid that melts at 48°C. It ignites readily and burns readily. The substance is insoluble in water and floats on the surface. Is the substance likely to be organic or inorganic?
(32) Give the molecular formulas for methylcyclopentane, 2-methylpentane, and cyclohexane. Which are strucutral isomers?
(33) The following names are incorrect. Write the bond-line structure for each compound as if it were correct and then give the correct IUPAC name.
- 2-dimethylpropane
- 2,3,3-trimethylbutane
- 2,4-diethylpentane
- 3,4-dimethyl-5-propylhexane
(34) Write equations for the complete combustion of each compound.
- propane (a bottled gas fuel)
- octane (a typical hydrocarbon in gasoline).
(35) The density of a gasoline sample is 0.690 g/mL. What is the weight in pounds for a 5.0 L container of gasoline?
(36) Draw the bond-line structures for the five isomeric hexanes (C6H14). Name each by the IUPAC system.
(37) Indicate whether the structures in each set represent the same compound or structural isomers.
(38) Consider the bond-line formulas shown below to answer the following questions.
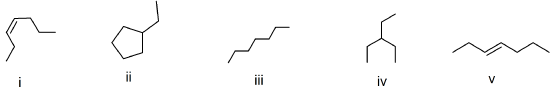
- Which compounds are structural isomers? Write the chemical formula(e) to support your answer(s).
- Which compounds are geometric isomers?