8.4: Titration
- Page ID
- 52180
Skills to Develop
- Explain what an acid/base indicator is.
- Explain how a titration is performed.
- Calculate the concentration of unknown acid or base when given the concentration of the other and the volume needed to reach the equilibrium point in a titration.
For acid-base neutralization reactions, the typical laboratory procedure for determining the stoichiometric amounts of acid and/or base in the reaction is to complete a titration. As we go through this section, we will use some of the prior knowledge we have obtained about acids and bases, chemical reactions, and molarity calculations, to apply them to the concept of titration.
Indicators
An indicator is a substance that changes color at a specific pH and is used to indicate the pH of the solution. Litmus paper is a paper that has been dipped in an indicator. The litmus paper is called an indicator because it is used to indicate whether the solution is an acid or a base. If the red litmus paper turns blue, the solution is basic (\(\text{pH} > 7\)); if the blue litmus turns red the solution is acidic (\(\text{pH} < 7\)).
 The juice from red cabbage can be used to prepare an indicator paper. It contains the chemical anthrocyanin, which is the active ingredient in the indicator. Red beets, blueberries, and cranberries are other great examples of naturally occurring indicators. Another example of a natural indicator is flowers. Hydrangea is a common garden plant with flowers that come in many colors depending on the pH of the soil. If you are traveling around and see a hydrangea plant with blue flowers, the soil is acidic; the creamy white flowers indicate the soil is neutral; and the pink flowers mean the soil is basic.
The juice from red cabbage can be used to prepare an indicator paper. It contains the chemical anthrocyanin, which is the active ingredient in the indicator. Red beets, blueberries, and cranberries are other great examples of naturally occurring indicators. Another example of a natural indicator is flowers. Hydrangea is a common garden plant with flowers that come in many colors depending on the pH of the soil. If you are traveling around and see a hydrangea plant with blue flowers, the soil is acidic; the creamy white flowers indicate the soil is neutral; and the pink flowers mean the soil is basic.
There are two requirements for a substance to function as an acid-base indicator: 1) the substance must have an equilibrium affected by hydrogen concentration, and 2) the two forms of the compound on opposite sides of the equilibrium must have different colors. Most indicators function in the same general manner and can be presented by a generic indicator equation. In the equation shown in the figure, we represent the indicator ion with a hydrogen ion attached as \(\ce{HIn}\) and we represent the indicator ion without the hydrogen attached as \(\ce{In^-}\).
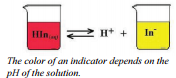 For this example, \(\ce{HIn}\) is red and \(\ce{In^-}\) is yellow. If we add hydrogen ion to the solution, the equilibrium will be driven toward the reactants and the solution will turn red. If we add base to the solution (reduce hydrogen ion concentration), the equilibrium will shift toward the products and the solution will turn yellow. It is important to note that if this indicator changes color at \(\text{pH} = 5\), then at all pH values less than 5, the solution will be red and at all pH values greater than 5, the solution will be yellow. Therefore, putting this indicator into a solution and
For this example, \(\ce{HIn}\) is red and \(\ce{In^-}\) is yellow. If we add hydrogen ion to the solution, the equilibrium will be driven toward the reactants and the solution will turn red. If we add base to the solution (reduce hydrogen ion concentration), the equilibrium will shift toward the products and the solution will turn yellow. It is important to note that if this indicator changes color at \(\text{pH} = 5\), then at all pH values less than 5, the solution will be red and at all pH values greater than 5, the solution will be yellow. Therefore, putting this indicator into a solution and 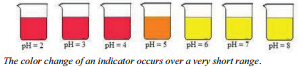 having the solution turn yellow does NOT tell you the pH of the solution . . . it only tells you that the pH is greater than 5 . . . it could be 6, 7, 8, 9, etc. There are many indicators that are available to be used to help determine the pH of solutions.
having the solution turn yellow does NOT tell you the pH of the solution . . . it only tells you that the pH is greater than 5 . . . it could be 6, 7, 8, 9, etc. There are many indicators that are available to be used to help determine the pH of solutions.
The Titration Process
One of the properties of acids and bases is that they neutralize each other to form water and a salt. In the laboratory setting, an experimental procedure where an acid is neutralized by a base (or vice versa) is known as titration. Titration, by definition, is the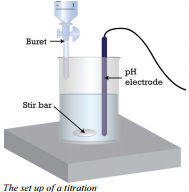 addition of a known concentration of base (or acid) to a solution of acid (or base) of unknown concentration. Since both volumes of the acid and base are known, the concentration of the unknown solution is then mathematically determined.
addition of a known concentration of base (or acid) to a solution of acid (or base) of unknown concentration. Since both volumes of the acid and base are known, the concentration of the unknown solution is then mathematically determined.
So what does one do in a titration? When doing a titration, you need to have a few pieces of equipment. A buret is used to accurately dispense the volume of the solution of known concentration (either the base or the acid). A flask is used to hold a known, measured volume of the unknown concentration of the other solution (either the acid or the base).
If the basic solution was in the buret, you would first read the volume of base in the buret at the beginning. You would add the base to the flask containing the acid until all of the acid has reacted and then read the volume of base in the buret again. To see how much was added, you would subtract the initial volume from the final volume.
In a titration, just enough base is added to completely react with all of the acid, without extra base being added. This is called the equivalence point because you have added equal moles of acid and base. For most acids and bases, this point is difficult to see, because the acid and base reactants as well as the salt and water products have no color. This is where indicators come in. An indicator is used to determine the equivalence of the titration. A few drops of the indicator are added to the flask before you begin the titration. If an appropriate indicator has been chosen, the indicator will only react and change color (and stay color changed) when all of the other acid has reacted. Therefore, the indicator will change color immediately after enough base was added to completely react with all of the acid (the equivalence point).
Some laboratories have pH meters that measures this point more accurately than the indicator, although an indicator is much more visual. The main purpose of a pH meter is to measure the changes in pH as the titration goes from start to finish. It is also possible to determine the equivalence point using the pH meter as the pH will change dramatically once all of the acid and base have been neutralized.
The Mathematics of Titration
For the calculations involved here, we will restrict our acid and base examples where the stoichiometric ratio of \(\ce{H^+}\) and \(\ce{OH^-}\) is 1:1. The formula for these 1:1 reactions, in which 1 mole of acid is needed to react with 1 mole of base, has the structure:
\[\left( \text{M}_a \right) \left( \text{V}_a \right) = \left( \text{M}_b \right) \left( \text{V}_b \right)\]
Where
- \(\text{M}_a\) is the molarity of the acid
- \(\text{V}_a\) is the volume of the acid
- \(\text{M}_b\) is the molarity of the base
- \(\text{V}_b\) is the volume of the base
This equation works because the left side calculates the number of moles of acid which react and the right side calculates the number of moles of base. To reach the equivalence point,
\[\text{mol acid} = \text{mol base}\]
Example 8.4.1
When \(10.0 \: \text{mL}\) of a \(0.125 \: \text{M}\) solution of hydrochloric acid, \(\ce{HCl}\), is titrated with a \(0.100 \: \text{M}\) solution of potassium hydroxide, \(\ce{KOH}\), what volume of the hydroxide solution is required to neutralize the acid?
Solution:
Step 1: Write the balanced ionic chemical equation. Check that the acid-base ratio is 1:1.
\[\ce{HCl} + \ce{KOH} \rightarrow \ce{H_2O} + \ce{KCl}\]
Since \( 1 \: \ce{HCl}\) is need for each \(\ce{KOH}\), the reaction is 1:1.
Step 2: Use the formula and fill in all of the given information. The acid is \(\ce{HCl}\) and the base is \(\ce{KOH}\).
\[\text{M}_a = 0.125 \: \text{M}\]
\[\text{V}_a = 10.0 \: \text{mL}\]
\[\text{M}_b = 0.100 \: \text{M}\]
\[\text{V}_b = ?\]
\[\left( \text{M}_a \right) \left( \text{V}_a \right) = \left( \text{M}_b \right) \left( \text{V}_b \right)\]
\[\left( 0.125 \: \text{M} \right) \left( 10.0 \: \text{mL} \right) = \left( 0.100 \: \text{M} \right) \left( \text{V}_b \right)\]
\[\text{V}_b = 12.5 \: \text{mL}\]
Therefore, for this strong acid-strong base titration, the volume of base required for the titration is \(12.5 \: \text{mL}\).
Lesson Summary
- An indicator is a substance that changes color at a specific pH and is used to indicate the pH of the solution.
- A titration is the addition of a known concentration of base (or acid) to a solution of acid (or base) of unknown concentration.
- The equivalence point is the point in the titration where the number of moles of acid equals the number of moles of base, and, if you chose an appropriate indicator, where the indicator changes color.
- For titrations where the stoichiometric ratio of \(\text{mol} \: \ce{H^+}\):\(\text{mol} \: \ce{OH^-}\) is 1:1, the formula \(\left( \text{M}_a \right) \left( \text{V}_a \right) = \left( \text{M}_b \right) \left( \text{V}_b \right)\) can be used to calculate concentrations or volumes for the unknown acid or base.
Vocabulary
- Titration: The lab process in which a known concentration of base (or acid) is added to a solution of acid (or base) of unknown concentration.
- Indicator: A substance that changes color at a specific pH and is used to indicate the pH of the solution.
- Equivalence point: The point in a titration where the number of moles of acid equals the number of moles of base.

