8.2: pH
- Page ID
- 52178
Skills to Develop
- State the \(\left[ \ce{H^+} \right]\), \(\left[ \ce{OH^-} \right]\), and \(K_w\) values for the self-ionization of water.
- Define and describe the pH scale and describe how logarithmic scales work.
- Calculate \(\left[ \ce{H^+} \right]\), \(\left[ \ce{OH^-} \right]\), and pH given the value of any one of the other values in a water solution at \(25^\text{o} \text{C}\).
- Explain the relationship between the acidity or basicity of a solution and the hydrogen ion concentration, \(\left[ \ce[H^+} \right]\), and the hydroxide ion concentration, \(\left[ \ce{OH^-} \right]\), of the solution.
- Predict whether an aqueous solution is acidic, basic, or neutral from the \(\left[ \ce{H^+} \right]\), \(\left[ \ce{OH^-} \right]\), or the pH.
Introduction
We have been discussing what makes an acid or a base and what properties acids and bases have. It is frequently useful to compare how acidic or basic a solution is in comparison to other solutions. A couple of ways to do this is to compare \(\left[ \ce{H^+} \right]\) to \(\left[ \ce{OH^-} \right]\) or to find the pH of a solution.
Relationship Between \(\left[ \ce{H^+} \right]\) and \(\left[ \ce{OH^-} \right]\)
We have learned that acids and bases are related to hydrogen ions \(\left[ \ce{H^+} \right]\) and hydroxide ions \(\left[ \ce{OH^-} \right]\). Both of these ions are present in both acids and bases. However, they are also present in pure water. Water self-ionizes according to the following reaction:
\[\ce{H_2O} \left( l \right) \rightleftharpoons \ce{H^+} \left( aq \right) + \ce{OH^-} \left( aq \right)\]
The equilibrium expression for this reaction would be:
\[K_w = \left[ \ce{H^+} \right] \left[ \ce{OH^-} \right]\]
The equilibrium constant for this particular equilibrium is \(K_w\), meaning the equilibrium constant for water. From experimentation, chemists have determined that in pure water, \(\left[ \ce{H^+} \right] = 1 \times 10^{-7} \: \text{M}\) and \(\left[ \ce{OH^-} \right] = 1 \times 10^{-7} \: \text{M}\). If you substitute these values into the equilibrium expression, you find that \(K_w = 1 \times 10^{-14}\). Any solution which contains water, even if other things are added, will shift to establish this equilibrium. Therefore, for any solution, the following relationship will always be true:
\[K_w = 1 \times 10^{-14} = \left[ \ce{H^+} \right] \left[ \ce{OH^-} \right] @ \: 25^\text{o} \text{C}\]
We can describe whether a solution is acidic, basic, or neutral according to the concentrations in this equilibrium.
- If \(\left[ \ce{H^+} \right] = \left[ \ce{OH^-} \right]\), the solution is neutral (such as in pure water)
- If \(\left[ \ce{H^+} \right] > \left[ \ce{OH^-} \right]\), the solution is acidic. This means that \(\left[ \ce{H^+} \right] > 1 \times 10^{-7} \: \text{M}\)
- If \(\left[ \ce{H^+} \right] < \left[ \ce{OH^-} \right]\), the solution is basic. This means that \(\left[ \ce{OH^-} \right] > 1 \times 10^{-7} \: \text{M}\).
We can use this equation to calculate the concentrations of \(\ce{H^+}\) and \(\ce{OH^-}\). Consider the following example.
Example 8.2.1
Suppose acid is added to some water, and \(\left[ \ce{H^+} \right]\) is measured to be \(1 \times 10^{-4} \: \text{M}\). What would \(\left[ \ce{OH^-} \right]\) be?
Solution:
Substitute what we know into the equilibrium expression:
\[K_w = 1 \times 10^{-14} = \left[ \ce{H^+} \right] \left[ \ce{OH^-} \right]\]
\[1 \times 10^{-14} = \left[ 1 \times 10^{4} \right] \left[ \ce{OH^-} \right]\]
To isolate \(\left[ \ce{OH^-} \right]\), divide both sides by \(1 \times 10^{-4}\). This leaves,
\[\left[ \ce{OH^-} \right] = 1 \times 10^{-10} \: \text{M}\]
Note that because \(\left[ \ce{H+} \right] > \left[ \ce{OH^-} \right]\), the solution must be acidic.
Suppose, on the other hand, something is added to the solution that reduces the hydrogen ion concentration, a base.
Example 8.2.2
If the final hydrogen ion concentration is \(1 \times 10^{-12} \: \text{M}\), we can calculate the final hydroxide ion concentration.
Solution:
\[K_w = 1 \times 10^{-14} = \left[ \ce{H^+} \right] \left[ \ce{OH^-} \right]\]
\[1 \times 10^{-14} = \left[ 1 \times 10^{-12} \right] \left[ \ce{OH^-} \right]\]
To isolate \(\left[ \ce{OH^-} \right]\), divide both sides by \(1 \times 10^{12}\). This leaves,
\[\left[ \ce{OH^-} \right] = 1 \times 10^{-2} \: \text{M}\]
Note that because \(\left[ \ce{H^+} \right] < \left[ \ce{OH^-} \right]\), the solution must be basic.
Using the \(K_w\) expression, anytime we know either the \(\left[ \ce{H^+} \right]\) or the \(\left[ \ce{OH^-} \right]\) in a water solution, we can always calculate the other one.
Example 8.2.3
What would be the \(\left[ \ce{H^+} \right]\) for a grapefruit found to have a \(\left[ \ce{OH^-} \right]\) of \(1.26 \times 10^{-11}\)? What is \(\left[ \ce{H^+} \right]\) and is the solution acidic, basic, or neutral?
Solution:
\[K_w = 1 \times 10^{-14} = \left[ \ce{H^+} \right] \left[ \ce{OH^-} \right]\]
\[1 \times 10^{-14} = \left[ \ce{H^+} \right] \left[ 1.26 \times 10^{-11} \right]\]
To isolate \(\left[ \ce{H^+} \right]\), divide both sides by \(1.26 \times 10^{-11}\). This leaves,
\[\left[ \ce{H^+} \right] = 7.94 \times 10^{-4} \: \text{M}\]
Also, the solution must be acidic because \(\left[ \ce{H^+} \right] > \left[ \ce{OH^-} \right]\)
pH Scale
A few very concentrated acid and base solutions are used as industrial chemistry and inorganic laboratory situations. For the most part, however, acid and base solutions that occur in nature, those in cleaning, and those used in organic or biochemistry applications are relatively dilute. Most of the acids and bases dealt with in laboratory situations have hydrogen ion concentrations between \(1.0 \: \text{M}\) and \(1.0 \times 10^{-14} \: \text{M}\). Expressing hydrogen ion concentrations in exponential numbers becomes tedious and is difficult for those not trained in chemistry. A Danish chemist named Søren Sørensen developed a shorter method for expressing acid strength or hydrogen ion concentration with a non-exponential number. He named his method pH. The p from pH comes from the German word potenz meaning "power or the exponent of". Sørensen's idea that the pH would be a simpler number to deal with in terms of discussing acidity level led him to a formula that relates pH and \(\left[ \ce{H^+} \right]\):
\[\text{pH} = -\text{log} \: \left[ \ce{H^+} \right]\]
If the hydrogen ion concentration is between \(1.0 \: \text{M}\) and \(1.0 \times 10^{-14} \: \text{M}\), the value of the pH will be between 0 and 14.
Example 8.2.4
Calculate the pH of a solution given that \(\left[ \ce{H^+} \right] = 0.01 \: \text{M}\).
Solution:
\[\text{pH} = -\text{log} \left( 0.01 \right)\]
\[\text{pH} = 2\]
Sometimes you will need to use a calculator.
Example 8.2.5
Calculate the pH of saliva with \(\left[ \ce{H^+} \right] = 1.58 \times 10^{-6} \: \text{M}\).
Solution:
\[\text{pH} = -\text{log} \left( 1.58 \times 10^{-6} \right)\]
\[\text{pH} = 5.8\]
If you are given \(\left[ \ce{OH^-} \right]\) it is still possible to find the pH, but it requires one more step. You must first find \(\left[ \ce{H^+} \right]\) and then use the pH equation.
Example 8.2.6
Calculate the pH of a solution with \(\left[ \ce{OH^-} \right] = 7.2 \times 10^{-4} \: \text{M}\).
Solution:
In order to find pH, we need \(\left[ \ce{H^+} \right]\).
\[K_w = 1 \times 10^{-14} = \left[ \ce{H^+} \right] \left[ \ce{OH^-} \right]\]
\[1 \times 10^{-14} = \left[ \ce{H^+} \right] \left[ 7.2 \times 10^{-4} \right]\]
To isolate \(\left[ \ce{H^+} \right]\), divide both sides by \(7.2 \times 10^{-4}\). This leaves,
\[\left[ \ce{H^+} \right] = 1.39 \times 10^{-11} \: \text{M}\]
We can now find the pH
\[\text{pH} = -\text{log} \left( 1.39 \times 10^{-11} \right)\]
\[\text{pH} = 10.9\]
The pH scale developed by Sørensen is a logarithmic scale, which means that a difference in 1 in pH units indicates a difference of a factor of 10 in the hydrogen ion concentrations. A difference of 2 in pH units indicates a difference of a factor of 100 in the hydrogen ion concentrations. Not only is the pH scale a logarithmic scale but by defining the pH as the negative \(\text{log}\) of the hydrogen ion concentration, the numbers on the scale get smaller as the hydrogen ion concentration gets larger. For example, \(\text{pH} = 1\) is a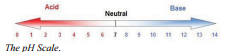 stronger acid than \(\text{pH} = 2\) and, it is stronger by a factor of 10 (the difference between the pH's is 1).
stronger acid than \(\text{pH} = 2\) and, it is stronger by a factor of 10 (the difference between the pH's is 1).
The closer the pH is to 0 the greater the concentration of \(\ce{H^+}\) ions and therefore the more acidic the solution. The closer the pH is to 14, the higher concentration of \(\ce{OH^-}\) ions and the stronger the base.
Have you ever cut an onion and had you eyes water up? This is because of a compound with the formula \(\ce{C_3H_6OS}\) that is found in onions. When you cut the onion, a variety of reactions occur that release a gas. This gas can diffuse into the air and mix with the water found in your eyes to produce a dilute solution of sulfuric acid. This is what irritates your eyes and causes them to water. There are many common examples of acids and bases in our everyday lives. Look at the pH scale to see how these common examples relate in terms of their pH.
Example 8.2.7
Compare lemon juice (\(\text{pH} = 2.5\)) to milk (\(\text{pH} = 6.5\)). Answer each of the following:
a) Label each as acidic, basic, or neutral
b) Which has a higher concentration of \(\ce{H^+}\) ions?
c) How many times more \(\ce{H^+}\) does that solution have?
Solution:
a) Both lemon juice and milk are acidic, because their pH's are less than 7. (*Note: milk is only very slightly acidic as its pH is very close to 7)
b) The lower the pH, the higher the concentration of \(\ce{H^+}\) ions. Therefore, lemon juice has more \(\ce[H^+}\).
c) Each step down on the pH scale increases the \(\ce{H^+}\) concentration by 10 times It is 4 steps down on the pH scale to go from 6.5 to 2.5. Therefore, lemon juice has \(10 \times 10 \times 10 \times 10\) 10,000 times more \(\ce{H^+}\) ions than milk.
Lesson Summary
- Water ionizes slightly according to the equation \(\ce{H_2O} \left( l \right) \rightleftharpoons \ce{H^+} \left( aq \right) + \ce{OH^-} \left( aq \right)\).
- The equilibrium constant for the dissociation of water is: \(K_w = 1 \times 10^{-14} = \left[ \ce{H^+} \right] \left[ \ce{OH^-} \right]\).
- \(\text{pH} = -\text{log} \left[ \ce{H^+} \right]\).

