IC3. Physical Properties
- Page ID
- 4062
The strong electrostatic forces of attraction of a cation for its surrounding anions, and the strong electrostatic forces of attraction of an anion for its surrounding cations, keep the compound together.
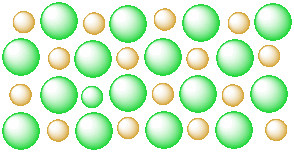
Figure IC3.1. A salt in the solid state. The ions are tightly packed together in an ordered arrangement.
Most of the time, we think of ionic compounds as solids. In the solid state, the ions are very close together. The forces of attraction between cations and anions are very high. In the liquid (melted) state, the ions would move around independently, and would be able to move a little farther apart from each other. In order to accomplish this independent movement, a great deal of energy would have to be supplied to get over the strong forces of attraction in the solid state. It takes a lot of heat to supply enough energy to convert them into liquid.

Figure IC3.2. A salt in the liquid (melted) state. The ions are still close together but more mobile and less ordered.
-
Ionic compounds often have high melting points.
In the gas phase, ions would be very far from each other and would move very freely. They would no longer attract each other very strongly because of the distances between ions. In order to convert an ionic compound into vapour, an enormous amount of energy would have to be supplied to overcome the attractions in the solid or liquid phase. Often, so much energy is supplied that they undergo decomposition into different compounds rather than boiling.
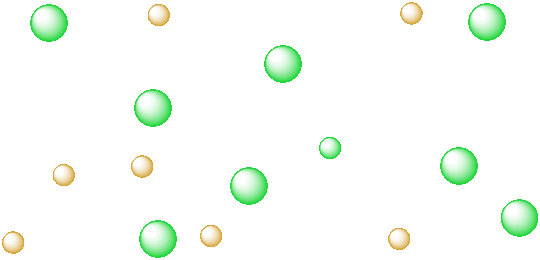
Figure IC3.3. A salt in the vapor state. The ions are far apart and randomly arranged.
- Ionic compounds have extremely high boiling points.
The changes of state in ionic compounds are governed by simple electrostatic forces between the ions. These electrostatic forces are governed by Coulomb's Law, in which the force of attraction depends on the amount of charge and the distance between the ions. That means that there are sometimes predictable variations in the properties of ionic compounds.
For example, among the potassium halides, the melting point is lowest for the iodide (681 oC) and highest for the fluoride (858 oC). The reason for that comes from the distance dependence in Coulomb's law.
Iodide is a bigger ion than bromide, chloride or fluoride. That means the distance between atoms is greater in potassium iodide than in potassium fluoride.In other words, the average distance between the positive potassium nucleus and the negative electrons surrounding the anion is greater for iodide than for chloride or fluoride. Note that this average distance really amounts to the distance between the two nuclei.
The greater the distance between ions, the lower the forces of attraction. The ions in potassium iodide can move around more easily than the ions in potassium fluoride. Potassium iodide has a lower melting point than potassium fluoride.
- Compounds containing smaller ions often have higher melting points than similar compounds that contain larger ions.
Problem IC3.1.
Select the compound that would have the lowest melting point in each of the following pairs.
a) KCl or LiCl b) NaF or NaBr c) CaO or BeO d) LiF or KBr
There is another factor, too: the force of attraction between ions also depends on the magnitude of the charges involved. The greater the size of the charge on an ion, the greater the force of attraction for its counterion.
For example, calcium fluoride, CaF2, has a lower melting point (1418 oC) than calcium oxide, CaO (2572 oC).
This difference is probably not due to differences in the distance between the charges. Fluoride and oxide are almost the same size, and if anything fluoride is a little smaller. Based on interionic distance alone, fluoride could have a slightly higher melting point than calcium oxide.
However, each fluoride has a 1- charge, but the oxide has a 2- charge. As a result of this greater charge, the force of attraction between an oxide and a calcium ion is stronger than the force of attraction between a fluoride and a calcium ion. It is more difficult to get the calcium and oxide ions to move away from each other, and the melting point is higher than for calcium fluoride.
- Compounds containing more highly charged ions often have higher melting points than similar compounds that contain lower charged ions.


