SP6. Hydrogen bonding
- Page ID
- 4152
SP6. Hydrogen Bonding
Methanol, CH4O (or CH3OH, a CH3 group attached to an OH), is another example of a molecule with a similar molecular weight to ethane. Like formaldehyde, methanol freezes somewhere around -90 oC, but it does not become a gas until it is heated to 65 oC. Methanol is certainly similar to formaldehyde in some ways. It contains oxygen and is very polar. The difference in their boiling points is due to the very strong hydrogen bonds in methanol.
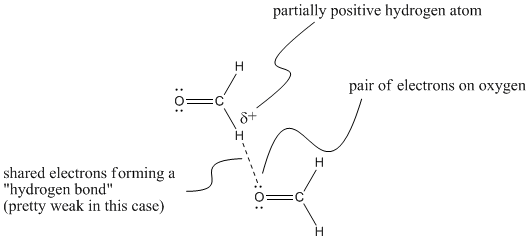
Hydrogen bonding is really a special case of dipole forces. It occurs under very particular circumstances. In general, a hydrogen bond is thought of as the electrostatic interaction between an electron pair on one atom and a hydrogen atom that has built up a partial positive charge. For example, a lone pair on an oxygen atom in formaldehyde might be attracted to a hydrogen on another formaldehyde, which would be at the more positive end of the molecule. There is evidence from x-ray crystallography that such weak hydrogen bonds occur in molecules similar to formaldehyde.
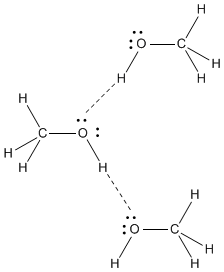
Very strong hydrogen bonds occur only when a hydrogen is attached to one of the few most electronegative elements in the periodic table: fluorine, oxygen or nitrogen. It is these strong hydrogen bonds that are responsible for the relatively high boiling point of methanol; there is so much positive charge on the hydrogen of the OH group that it can essentially form a real bond with the lone pair on another methanol molecule.
Water is an even better example of a hydrogen bonding compound; in fact, water molecules are so tightly bound to each other that this very small molecule freezes at 0 oC and does not boil until 100 oC (way, way hotter than an August day in Las Vegas). Water molecules are much less able to move around freely than are ethane molecules, even though ethane molecules weigh almost twice as much.
Problem SP6.1.
Which of the following molecules are capable of strong hydrogen bonding?
a) ethanal, CH3CHO b) ethylamine, CH3CH2NH2 c) acetic or ethanoic acid, CH3CO2H d) dimethyl peroxide, CH3OOCH3
Problem SP6.2.
Predict the relative order of melting points in the following amines:
hexylamine, CH3(CH2)4CH2NH2; triethylamine, (CH3CH2)3N; dipropylamine, (CH3CH2CH2)NH
Biological Molecules and IMFs
Large biological molecules like proteins consists of 1000s of atoms, which interact through "intramolecular" ion-ion, van der Waals, dipole-dipole, and hydrogen bonding to create a unique 3D structure. Other molecules, both small and large, can bind to biological macromolecule using the same intermolecular interactions. An example of a protein, bovine low molecular weight protein tyrosyl phosphate, binding to inorganic phosphate, is shown below. Select the radio buttons below to alter the rendering of the molecular and obtain a better understanding of the structure/activity relationships of the protein:
| spacefill | wireframe | cartoon |
| The displays above show three different ways to render the LMW-PTPase. You will recognize the spacefill and wireframe models, but note their complexity compared to the smaller molecules you have studied thus far. The cartoon molecules removes much of the detail by stripping away atoms and bonds, and replacing them with lines, ribbons (representing alpha helices) and block arrows (representing beta structure). | ||
| cartoon with phosphate | wireframe with phosphate | |
| Inorganic phosphate, an inhibitor of this enzyme, binds to the enzyme through the intramolecular forces mentioned above. It is rendered in spacefill with the protein in cartoon and wireframe modes. | ||