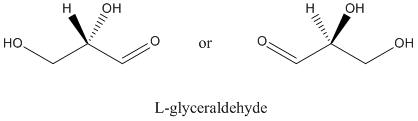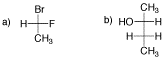SC8. Biological Building Blocks: Carbohydrates
- Page ID
- 4133
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
SC8. Biological Building Blocks: Carbohydrates
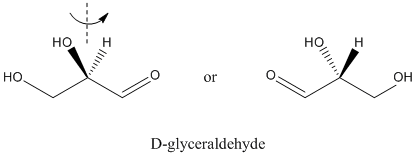
- The naturally-occurring carbohydrates are chiral.
- Glyceraldehyde is the grandmother of carbohydrates.
| | Wire Frame Ball & Stick Spacefilling |
| | Wire Frame Ball & Stick Spacefilling |
- The "last" chiral center in other carbohydrates all have the same configuration as the chiral center in glyceraldehyde.
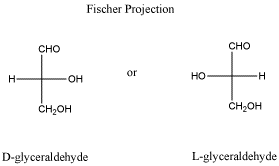
- Fischer projections are drawings that are sometimes used to show stereochemistry in a different way.
- The groups to left and right of the Fischer projection are coming towards you.
- The groups on top and bottom are going away from you.
- The carbon chain is drawn vertically with attachments at the sides.
- Normally, the chain is oriented so that the "highest priority functional group" on the chain is at or near the top of the chain; that usually means the group with the most bonds to oxygen. In glyceraldehyde, the CH=O group is drawn at the top rather than at the bottom.
Problem SC8.1.
What is the absolute configuration of L-glyceraldehyde? What about D-glyceraldehyde?