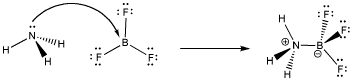AB4. Lewis Acid-Base Complexes
- Page ID
- 4022
AB4. Lewis Acid-Base Complexes
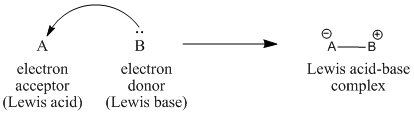
What happens when a Lewis base donates a pair of electrons to a Lewis acid? The arrow formulism we have been using to illustrate the behaviour of Lewis acids and Lewis bases is meant to show the direction of electron movement from the donor to the acceptor. However, given that a bond can be thought of as a pair of electrons that are shared between two atoms (in this case, between the donor and the acceptor), these arrows also show where bonds are forming.
Figure AB4.1. Donation of electrons from a Lewis base to a Lewis acid.
The electrons donated from a Lewis base to a Lewis acid form a new bond. A new, larger compound is formed from the smaller Lewis acid and Lewis base. This compound is called a Lewis acid-base complex.
A simple example of Lewis acid-base complexation involves ammonia and boron trifluoride. The nitrogen atom has a lone pair and is an electron donor. The boron has no octet and is an electron acceptor. The two compounds can form a Lewis acid-base complex or a coordination complex together.
Figure AB4.2. Formation of a Lewis acid-base complex from ammonia and boron trifluoride.
When the nitrogen donates a pair of electrons to share with the boron, the bond that forms is sometimes called a coordinate bond. Another term for this kind of bond is a dative bond. A coordinate or dative bond is any covalent bond that arose because one atom brought a pair of its electrons and donated them with another.
There is another piece of terminology you should get used to here. Sometimes, the electron donor is called a nucleophile and the electron acceptor is called an electrophile. Ammonia is a nucleophile and boron trifluoride is an electrophile.
- Because Lewis bases are attracted to electron-deficient atoms, and because positive charge is generally associated with the nucleus of an atom, Lewis bases are sometimes refered to as "nucleophiles". Nucleophile means nucleus-loving.
- Because Lewis acids attract electron pairs, Lewis acids are sometimes called "electrophiles". Electrophile meanse electron-loving.
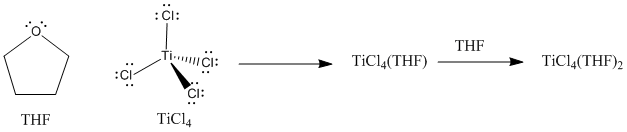
Lewis acid-base complexes frequently have very different properties from the separate compounds from which they were formed. For example, titanium tetrachloride is a yellow liquid at room temperature. It is so Lewis acidic that it reacts with moisture in the air, undergoing a reaction that generates HCl gas in the form of white smoke. Tetrahydrofuran (or THF), a mild Lewis base, is a colourless liquid. When THF and TiCl4 are combined, a Lewis acid-base complex is formed, TiCl4(THF)2. TiCl4(THF)2 is a yellow solid at room temperature. Although it still reacts with the air, it does so very slowly, and shows no visible change when exposed to the air for several minutes.
Figure AB4.5. A Lewis acid-base complex between tetrahydrofuran (THF) and titanium tetrachloride.
Problem AB4.1.
Show, using arrow notation, the reaction between THF and titanium tetrachloride to form the Lewis acid-base complex, TiCl4(THF)2. Also show the structures of the complexes formed.
Problem AB4.2.
A similar Lewis acid-base complex is formed between THF and borane, BH3.
- Which compound is the Lewis acid? Which one is the Lewis base?
- Which atom in the Lewis acid is the acidic site? Why?
- Which atom in the Lewis base is the basic site? Why?
- How many donors would be needed to satisfy the acidic site?
- Show, using arrow notation, the reaction to form a Lewis acid-base complex.
- Borane is highly pyrophoric; it reacts violently with air, bursting into flames. Show, using arrow notation, what might be happening when borane contacts the air.
- Borane-THF complex is much less pyrophoric than borane. Why do you suppose that is so?
Problem AB4.3.
When a neutral Lewis acid combines with an anionic Lewis base, the product is called a complex ion. The same is true if a cationic Lewis acid combines with a neutral Lewis base.
Show the formation of the following polyatomic anions from the Lewis acid-base pairs that were combined in each case.
a) BF4- b) PF6- c) AlCl4- d) AlH4- e) Ag(NH3)2+