AB3. Lewis Acids
- Page ID
- 4021
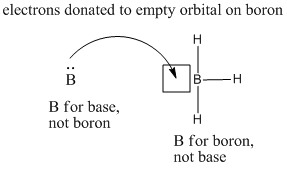
Borane is unusual because it is a compound without an octet. The central boron atom has only six valence electrons. It needs one more pair of electrons to obtain an octet. The boron is a Lewis acid.
Figure AB3.1. Borane is a Lewis acid. It can accept electrons from a donor atom. The square drawn beside the boron is used to reinforce the idea that there is a vacant site for electrons there.
- Lewis acids are often short of a complete octet.
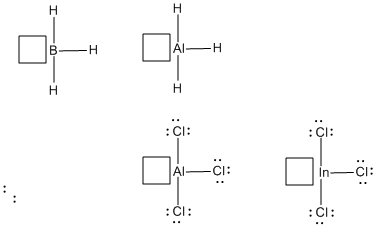
In the main group of the periodic table, atoms in the Group IIIA column (including boron and aluminum) have three valence electrons to share in order to make bonds. Sharing these electrons with three electrons from neighbors would make three bonds, and provide six electrons, not eight, in the valence shell. Another pair of electrons must be accepted from a donor to achieve an octet.
Figure AB3.2. Boron, aluminum and indium are from the same column of the periodic table. All three are often Lewis acidic; they can accept electrons from donors.
- Boron, aluminum and indium compounds are often Lewis acids.
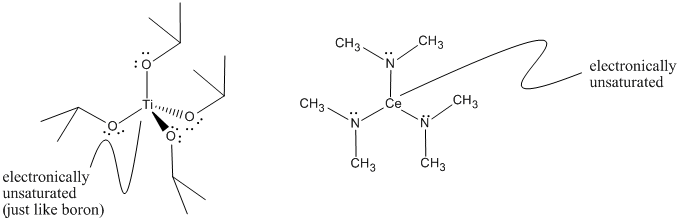
The eight-electron rule does not hold throughout the periodic table. In order to obtain noble gas configurations, some atoms may need eighteen electrons in their valence shell. For example, transition metals such as titanium often follow an eighteen-electron rule. Titanium has four valence electrons and can form four bonds in compounds such as titanium tetrakis (isopropoxide), below, or titanium tetrachloride, TiCl4. However, the titanium atom in that compound has only eight valence electrons, not eighteen. It can easily accept electrons from donors.
Figure AB3.3. Although titanium has eight electrons in this molecule, titanium tetrakis(isopropoxide), it can accommodate up to eighteen. It is Lewis acidic. The cerium atom in cerium tris(dimethylamide) comes from a similar part of the periodic table and is also Lewis acidic.
- Transition metals such as titanium, iron and nickel may have up to eighteen electrons and can frequently accept electron pairs from Lewis bases. Transition metals are often Lewis acids.
- Lanthanides such as cerium and samarium could conceivably have up to thirty-two electrons in their valence shells! They never do. However, they are usually strong Lewis acids.
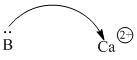
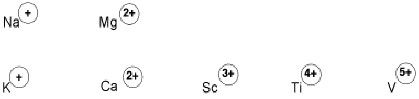
Positive ions are often Lewis acids because they have an electrostatic attraction for electron donors. Examples include alkali and alkaline earth metals in the group IA and IIA columns. K+, Mg2+ and Ca2+ are sometimes seen as Lewis acidic sites in biology, for example. These ions are very stable forms of these elements because of their low electron ionization potentials. However, their positive charges do attract electron donors.
Figure AB3.4. A calcium ion essentially has a noble gas configuration. Nevertheless, its positive charge can attract electrons from a donor atom.
In a similar way, "early" transition metals -- those that are close to the left hand side of the periodic table, especially in groups IIIB, IVB and VB -- have low ionization potentials and have high positive charges or oxidation states. For example, Sc3+, Zr4+ and V5+ are common forms of some early transition metals, and they are strong Lewis acids.
Figure AB3.5. A few alkali, alkaline earth and transition metals that are commonly found as cations.
-
Many cations such as Ca2+ or Sc3+ are good Lewis acids. Their positive charges attract electrons.
Sodium, potassium, calcium and magnesium are common ions in biology. Sodium, potassium and calcium have well-known roles in cell signaling. The build-up of these ions on one side or another of a cell membrane results in a charge separation across the membrane, called a membrane potential. However, the Lewis acidity of these ions are also important in biology. Because they attract electron donors, these ions are generally found in biological situations with water molecules stuck to them; remember that the oxygen atom in water is Lewis basic. That means that the proteins that form ion channels in cell membranes must somehow cope with the water molecules that are all around when they are transporting potassium ions or sodium ions across the membranes. A potassium ion covered in water molecules is a much larger object than a potassium ion by itself. The pore that opens up between the proteins must be big enough to allow the entire water-potassium complex through, or else there must be a way to separate the water molecules from the potassium ion. At the same time, the ion channel may exploit the Lewis acidity of the potassium ion; for example, the channel proteins may contain oxygen atoms situated in such a way that they draw the ion into the channel. Exactly how ion channels work is a very active topic of current research at the interface of chemistry and biology.
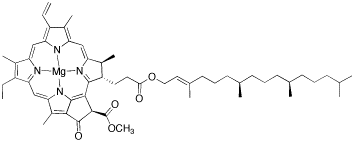
There are other biological situations in which the Lewis acidity of these ions plays a role. For example, potassium ions bind to nucleic acids in DNA to form the telomeres at the ends of chromosomes. This binding involves the donation of lone pairs on the nucleic acids (guanidines in this case) to a potassium ion. Another common biological structure in which donor atoms bind to a Lewis acid is chlorophyll, in which a porphyrin ring binds to a central magnesium ion. Chlorophyll is involved in the absorption of sunlight and the initiation of electron transport to photosystem I and II, which are the engines of photosynthesis. One of the roles of the magnesium ion in this structure is to affect the color of the chlorophyll and hence the wavelengths of light absorbed; when other metal ions are bound by the porphyrin, different colors are absorbed.
Figure AB3.6. Chlorophyll A.
A number of biological molecules that contain metal ions perform tasks that require Lewis acidity. Many enzymes that contain metal ions, sometimes called metalloproteins, bind a substrate and carry out a transformation on it. The substrate is usually able to donate a pair of electrons to the metal ion and stick to it while a reaction occurs. For example, vanadium bromoperoxidase is a protein found in some kinds of marine algae. It can bind dissolved oxygen molecules as well as bromide ions from the surrounding saltwater. A subsequent series of reactions allows the bromine atom to be incorporated into large organic molecules. Halogens in organic molecules are extremely rare in nature, but it is perhaps not surprising that organisms surrounded by salt water have managed to make them. The resulting brominated compounds seem to be used as chemical defenses, making the algae less likely to get eaten.
A number of other metal ions are common in biology. Manganese is prominent in photosystem I and II, iron is found in hemoglobin, and cobalt is in vitamin B12. Hemoglobin may be familiar because of its role in transporting oxygen in the blood.
Figure AB3.7. Some transition metal ions that are common in biology.
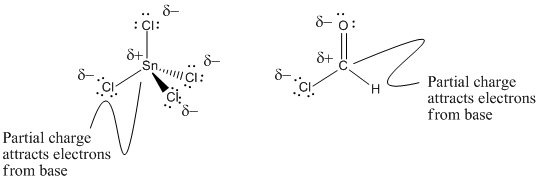
Atoms in the lower part of the periodic table often have variable oxidation states. They can form different numbers of bonds. Although carbon generally forms four bonds, forming compounds like carbon tetrachloride, CCl4, tin can sometimes form two bonds as well as four. Tin forms stannous chloride, SnCl2, as well as stannic chloride, SnCl4. It is possible that stannous chloride, with sixteen valence electrons, could accept another pair of electrons.
It may be surprising that stannic chloride is also a Lewis acid. The tin atom in stannic chloride has a full octet (eighteen valence electrons), but it still attracts electron donors. Given the difference in electronegativity between tin and chlorine, however, it seems reasonable to think of these compounds in terms of very polar bonds.
Figure AB3.8. Tin and carbon atoms in environments that make them attract electrons.
- Atoms with an octet can still be Lewis acidic if they bear a large, partial positive charge.
Carbon atoms can be Lewis acidic even though carbon does not generally form ionic bonds. For example, formyl chloride, H-(C=O)-Cl, contains a carbon atom that is bonded to a chlorine and also double bonded to an oxygen atom. Although these bonds are certainly covalent, bond polarity places a large, partial positive charge on the carbon. Formyl chloride easily attracts a pair of electrons from a Lewis base.
Problem AB3.1.
Which of the following compounds appear to be Lewis acids? Explain your reasoning in each case.
a) CeCl3 b) BF3 c) CH4 d) CH2O e) N2