AB2. Lewis Bases
- Page ID
- 4020
What makes a molecule (or an atom or ion) a Lewis base? It must have a pair of electrons available to share with another atom to form a bond. The most readily available electrons are those that are not already in bonds. Bonding electrons are low in energy. Non-bonding electrons are higher in energy and may be stabilized when they are delocalized in a new bond.
Lewis bases usually have non-bonding electrons or lone pairs.
| Example 1: Ammonia |
|---|
| Ammonia, NH3, has a lone pair and is a Lewis base. It can donate to compounds that will accept electrons. Figure AB3.1. Ammonia donating to an electron acceptor or Lewis acid. |
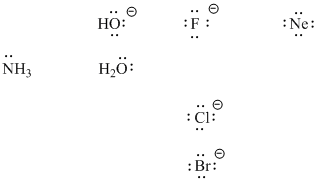
Lewis bases may be anionic or neutral. The basic requirement is that they have a pair of electrons to donate. Examples of Lewis bases include halide ions such as bromide or chloride. To the right of the halides in the periodic table are Noble gases such as neon. Noble gases do have lone pairs, but are stable enough that they do not usually react. They are not very good Lewis bases. To the left of the halides, however, are other examples in oxygen and nitrogen compounds. Water also has lone pairs and is a common Lewis base, and so is hydroxide ion, HO-.
Figure AB3.2. Some examples of Lewis basic ions and molecules. Note that neon, although it has nonbonding electron pairs or lone pairs, does not usually act as a Lewis base.
- Halides, water, ammonia and hydroxide ion are examples of Lewis bases.
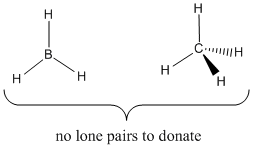
One column further to the left in the periodic table from nitrogen is carbon. Carbon does not normally have a lone pair. For example, methane, CH4, has all of its valence electrons in bonding pairs. These bonding pairs are too stable to donate under normal conditions. Methane is not a Lewis base.
Figure AB3.3. Carbon and boron "hydrides". Neither of these compounds has a lone pair, and neither is a good Lewis base.
Even further to the left is boron. A simple boron compound is borane, BH3. Borane has no lone pairs; all its valence electrons are in bonds. Boron is not a good Lewis base.
Problem AB3.1.
Which of the following compounds appear to be Lewis bases?
a) SiH4 b) AlH3 c) PH3 d) SH2 e) -SH