4.7 Conformations of Monosubstituted Cyclohexanes
- Page ID
- 44201
Objectives
After completing this section, you should be able to
- account for the greater stability of the equatorial conformers of monosubstituted cyclohexanes compared to their axial counterparts, using the concept of 1,3-diaxial interaction.
- compare the gauche interactions in butane with the 1,3-diaxial interactions in the axial conformer of methylcyclohexane.
- arrange a given list of substituents in increasing or decreasing order of 1,3-diaxial interactions.
Key Term
Make certain that you can define, and use in context, the key term below.
- 1,3-diaxial interaction (see the “Study Notes,” below)
Study Notes
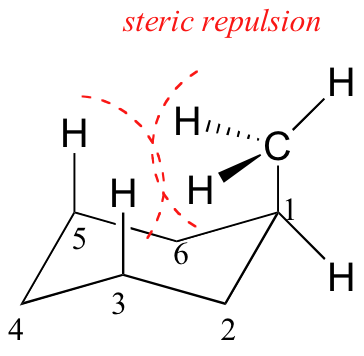
1,3-Diaxial interactions are steric interactions between an axial substituent located on carbon atom 1 of a cyclohexane ring and the hydrogen atoms (or other substituents) located on carbon atoms 3 and 5. Compare these interactions with the across-the-ring interactions in cyclobutane referred to in Problem 4.11 (p. 118 of the textbook). Be prepared to draw Newman-type projections for cyclohexane derivatives as shown in Figure 4.14 on page 125 of the textbook. As usual, you will find that molecular models are invaluable aids to achieving this objective. We suggest that you start by making a model of methylcyclohexane. You can now observe how close the methyl hydrogens are to the axial hydrogens attached to carbons 3 and 5. Next, sight along the C1-C2 bond, holding the model in such a way that the ring-methyl C-C bond is pointing upwards. The “Newman projection” of C1 can now be drawn.
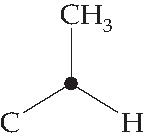
Figure 4.1: Newman projection of C1 in methylcyclohexane
To complete the projection, you must show the atoms that are attached to carbon atom 2, just as you did in Unit 3 when you were drawing Newman projections for the simple alkanes. After looking along the C1 C2 bond of your model you should be able to draw the following diagram.
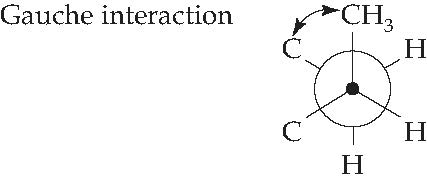
Figure 4.2: Newman projection of the C1 C2 bond of methylcyclohexane
The gauche interaction between the methyl substituent and the ring methylene (CH3) group is quite clear from this diagram. You may now add additional detail, as shown in Figure 4.14, if you wish.
Because axial bonds are parallel to each other, substituents larger than hydrogen generally suffer greater steric crowding when they are oriented axial rather than equatorial. Consequently, substituted cyclohexanes will preferentially adopt conformations in which the larger substituents assume equatorial orientation.
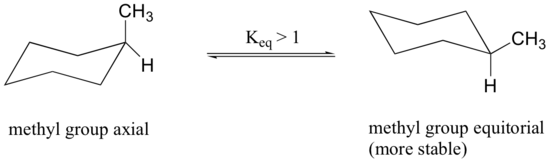
When the methyl group in the structure above occupies an axial position it suffers steric crowding by the two axial hydrogens located on the same side of the ring.
The conformation in which the methyl group is equatorial is more stable, and thus the equilibrium lies in this direction.
The relative steric hindrance experienced by different substituent groups oriented in an axial versus equatorial location on cyclohexane may be determined by the conformational equilibrium of the compound. The corresponding equilibrium constant is related to the energy difference between the conformers, and collecting such data allows us to evaluate the relative tendency of substituents to exist in an equatorial or axial location.A table of these free energy values (sometimes referred to as A values) may be examined by clicking here.
Looking at the energy values in this table, it is clear that the apparent "size" of a substituent (in terms of its preference for equatorial over axial orientation) is influenced by its width and bond length to cyclohexane, as evidenced by the fact that an axial vinyl group is less hindered than ethyl, and iodine slightly less than chlorine.
We noted earlier that cycloalkanes having two or more substituents on different ring carbon atoms exist as a pair (sometimes more) of configurational stereoisomers. Now we must examine the way in which favorable ring conformations influence the properties of the configurational isomers. Remember, configurational stereoisomers are stable and do not easily interconvert, whereas, conformational isomers normally interconvert rapidly. In examining possible structures for substituted cyclohexanes, it is useful to follow two principles:
- Chair conformations are generally more stable than other possibilities.
- Substituents on chair conformers prefer to occupy equatorial positions due to the increased steric hindrance of axial locations.
| Substituent | \(-\Delta{G}^o\) kcal/mol | Substituent | \(-\Delta{G}^o\) kcal/mol |
| \(\ce{CH_3\bond{-}}\) | 1.7 | \(\ce{O_2N\bond{-}}\) | 1.1 |
| \(\ce{CH_2H_5\bond{-}}\) | 1.8 | \(\ce{N#C\bond{-}}\) | 0.2 |
| \(\ce{(CH_3)_2CH\bond{-}}\) | 2.2 | \(\ce{CH_3O\bond{-}}\) | 0.5 |
| \(\ce{(CH_3)_3C\bond{-}}\) | \(\geq 5.0\) | 0.7 | |
| \(\ce{F\bond{-}}\) | 0.3 | 1.3 | |
| \(\ce{Cl\bond{-}}\) | 0.5 | \(\ce{C_6H_5\bond{-}}\) | 3.0 |
| \(\ce{Br\bond{-}}\) | 0.5 | ||
| \(\ce{I\bond{-}}\) | 0.5 |
Chlorocyclohexane
This is an example of the next level of complexity, a mono-substituted cycloalkane. See Fig 3.
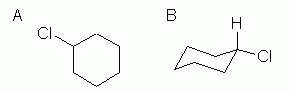
Figure 4.7.3:
So what is new here? Not much, with the hexagon formula, Fig 3A. That type of formula shows the basic "connectivity" of the atoms -- who is connected to whom. This chemical has one Cl on the ring, and it does not matter where we show it. There is now only one H on that C, but since we are not showing H explicitly here, that is not an issue in drawing the structure. (It is an issue when you look at it and want to count H.)
With the chair formula (Fig 3B), which shows information not only about connectivity but also about conformation, there is important new information here. In a chair, there are two "types" of substituents: those pointing up or down, and called axial, and those pointing "outward", and called equatorial. I have shown the chlorine atom in an equatorial position. Why? Two reasons: it is what we would predict, and it is what is found. Why do we predict that the Cl is equatorial? Because it is bigger than H, and there is more room in the equatorial positions.
Helpful Hints
If possible, examine a physical model of cyclohexane and chlorocyclohexane, so that you can see the axial and equatorial positions. Common ball and stick models are fine for this. It should be easy to see that the three axial H on one side can get very near each other.
If you do not have access to physical models, examining computer models can also be useful. When putting substituents on chair structures, I encourage you to use the four corner positions of the chair as much as possible. It is easier to see the axial and equatorial relationship at the corners.
In Fig 3B I have shown the H atom that is on the same carbon as the Cl atom. This is perhaps not necessary, since the correct number of H atoms is understood, by counting bonds on C. But showing the H explicitly at key C atoms helps to make the structure clearer. This may be particularly important with hand-drawn structures. I often see structures where I am not sure whether a particular atom is shown axial or equatorial. But if both atoms at the position (the H as well as the Cl) are shown, then hopefully it becomes clearer which is which. I also encourage students who are not sure of their art work to annotate their drawing. Say what you mean. That allows me to distinguish whether you are unsure which direction things point or simply unsure how to draw them.)
For notes on how to draw chairs (by hand or using a drawing program), see the section E.2. Note: How to draw chairs.
Contributors
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
>Robert Bruner (http://bbruner.org)
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)

