Synthesis of Aldehydes & Ketones
- Page ID
- 55196
Aldehydes and ketones can be prepared using a wide variety of reactions. Although these reactions are discussed in greater detail in other sections, they are listed here as a summary and to help with planning multistep synthetic pathways. Please use the appropriate links to see more details about the reactions.
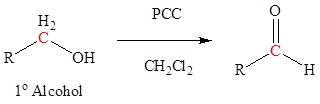
Oxidation of 1o alcohols with PCC to form aldehydes
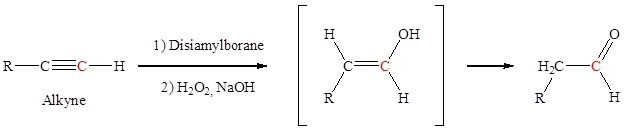
Hydration of an alkyne to form aldehydes
Anti-Markovnikov addition of a hydroxyl group to an alkyne forms an aldehyde. The addition of a hydroxyl group to an alkyne causes tautomerization which subsequently forms a carbonyl.
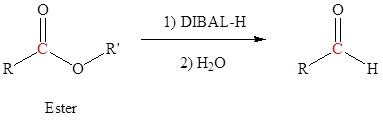
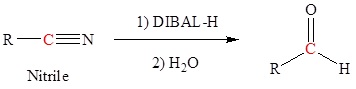
Reduction of an ester, acid chloride or nitrile to form aldehydes
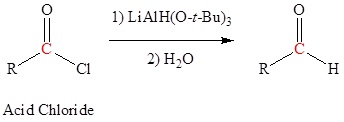
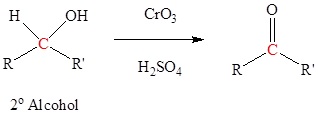
Oxidation of 2o alcohols to form ketones
Typically uses Jones reagent (CrO3 in H2SO4) but many other reagents can be used
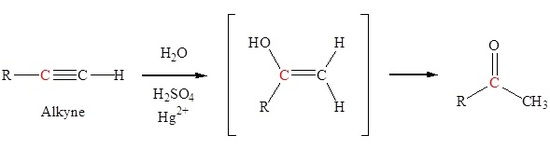
Hydration of an alkyne to form ketones
The addition of a hydroxyl group to an alkyne causes tautomerization which subsequently forms a carbonyl. Markovnikov addition of a hydroxyl group to an alkyne forms a ketone.
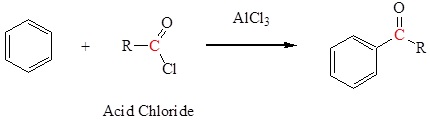
Friedel-Crafts acylation to form a ketone
Reaction of Grignard reagents with nitriles to form ketones
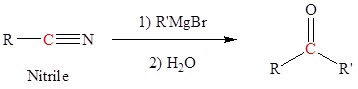
Alkenes can be cleaved using ozone (O3) to form aldehydes and/or ketones
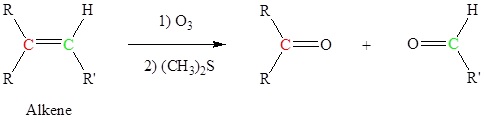
This is an example of a Ozonolysis reaction.
Contributors
Prof. Steven Farmer (Sonoma State University)

