9: Kinetics & Collision Theory
- Page ID
- 143999
Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Thermodynamic Review
Review Thermodynamics*
-
Fill in the blanks on the following paragraph about thermodynamics of chemical reactions.
The breaking of bonds requires __________ (input or release of energy) to break the attractive forces holding the atoms together, so bond breaking is _____________(endothermic/exothermic).
When bonds are formed, energy is _____________(required/released). Thus, the making of bonds is _____________(endothermic/exothermic).
Entropy generally __________ (increases/decreases) when a reaction produces more molecules than it started with.
Entropy generally __________ (increases/decreases) when a reaction produces fewer molecules than it started with.
In chemical reactions bonds are made and broken. Whether the overall reaction is spontaneous depends on two main factors: __________ and __________ .
-
For a process that occurs at a constant temperature, the change in free energy (∆G) is given by the equation:
-
Thus, ∆G is dependent on two key factors:
-
Complete the following with < or > or =:
If ∆G 0, the reaction is spontaneous (exergonic)
If ∆G 0, the reaction is non-spontaneous (endergonic)
If ∆G 0, the reaction mixture is at equilibrium
-
Generalization: DG < kJ mol-1 is irreversible.
-
Generalization: K > ______
is product favored.
*Answers in Canvas
Predicting Reaction Outcomes: Is DG all we need?
The standard free energy change, DGo, for the following reaction is -2.9 kJ/mol.
C (diamond) -> C (graphite)
-
Draw a reaction progress diagram for this process. (What would you guess would be true of the energy of activation (Ea) for the reaction? Think about what has to happen to the bonds in order for this conversion to occur.)
- This reaction is spontaneous, yet how likely are diamonds to turn into graphite?
A spontaneous process does not necessarily occur rapidly.
- Explore the following simulation (vary the temperature, the Ea, the DG): http://phet.colorado.edu/en/simulati...ible-reactions
Activation Energy affects Rate of Reaction:
Consider these two reactions that have different activation energies (Ea) occurring at the same initial temperature;
Reaction1: Y(aq) +P(aq) -> C(aq) Ea =92kJ
Reaction 2: T(aq) + V(aq) à Z(aq) Ea = 480 kJ
-
Explain in your own words what you understand by the term “activation energy”.
-
Which reaction do you think is likely to be faster? Explain your answer as fully as you can.
-
With the information provided, could you predict the extent of the reaction? (ie product-favored, reactant-favored or similar amounts of product and reactants).
Summary of Thermodynamics vs Kinetics
While thermodynamics determines the extent to which reactions occur, chemical kinetics is concerned with the rate of a chemical reaction. The reaction rate or speed of reaction for a reactant or product in a particular reaction is defined as how fast or slow a reaction takes place.
Collision Theory
Adapted from G. Cakmakci, J. Chem. Educ., 2010, 87, 449–455
Reaction Collisions
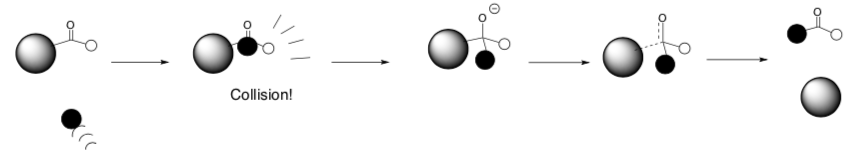
- Draw a reaction profile for the reaction above.
-
Label each step with a cartoon of what is happening. Remember that Transition States are at the top of a peak and represent the bond-making and bond- breaking steps.
-
If the Nu (
 ) doesn’t hit the carbon of the carbonyl in the right place, what will happen?
) doesn’t hit the carbon of the carbonyl in the right place, what will happen? -
If the Nu (
 ) barely taps the carbon of the carbonyl, what will happen?
) barely taps the carbon of the carbonyl, what will happen? -
What factors will determine which step will be the rate determining step (the step of the mechanism that has the highest Ea)?
Concentration and Rate of Reaction
-
The hydride concentration is higher in which round-bottom flask?
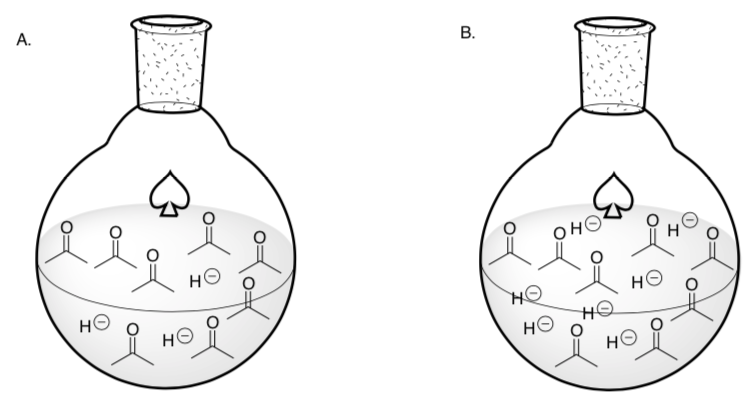
-
Draw the reaction that will occur in these flasks.
Collision Theory suggests that more collisions/interactions between reactants occur at higher concentrations.
-
As concentration of reactants increases,
The number of collisions _______________
And the rate of the reaction is ______________
-
Based on collision theory, which round-bottom reaction will occur faster?
A or B
Concentration Changes during the Reaction
For the following reaction: A + B -> C
-
Label which line in the graph below correlates to the concentration of reactant A.
-
Label which line in the graph below correlates to [C].
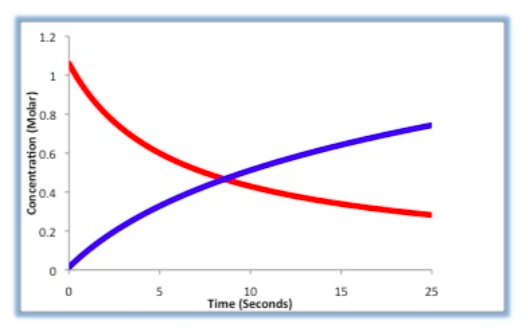
Concentration Problem
Tudor brand kettle de-scaler contains a 3% solution of acid. A different kettle de-scaler called Apex, containing a 5% solution of acid. When a student used the new kettle de- scaler for removing limestone collected in the kettle she realized that the new de-scalerApex removed the limestone faster than Tudor did.
-
Why does it take less time to remove limestone in the kettle with concentrated kettle de-scaler (Apex)? Explain your answer as fully as you can in terms of particles.
Temperature and Rate of Reaction
The kinetic theory of gases describes a gas as a large number of small particles (atoms or molecules), all of which are in constant, random motion. The rapidly moving particles constantly collide with each other and with the walls of the container.
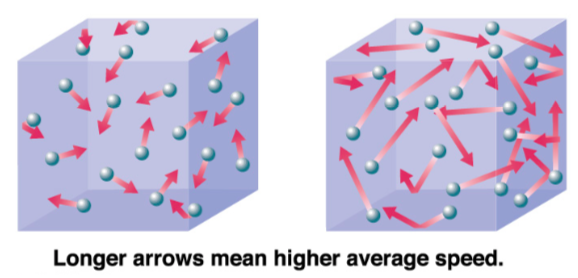
Molecules at a higher temperature have more thermal energy.
-
Molecules with higher thermal energy, have (more / less) collisions.
-
Bonds are (easier / harder) to break in molecules with higher thermal energy.
-
In a mixture of molecules at higher temperature, the proportion of reactant molecules with sufficient energy to react is (higher / lower).
When a house was newly built both the hot and the cold water pipes in the kitchen were shiny. After a while, the outside of these pipes had become dull and rusty (covered with a thin, brown coating). The outside of the hot water pipe was more rusty than the outside of the cold water pipe.
-
Explain why the outside of the hot water pipe was more rusty than the outsideof the cold water pipe. Please give as much detail as you can!
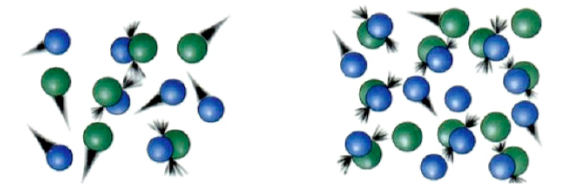
Pressure and Rate of Reaction
The following gaseous reaction occurs at room temperature (298 K):
A(g) +B(g) -> C(g) +D(g)
The reaction is set up under two different sets of initial conditions:
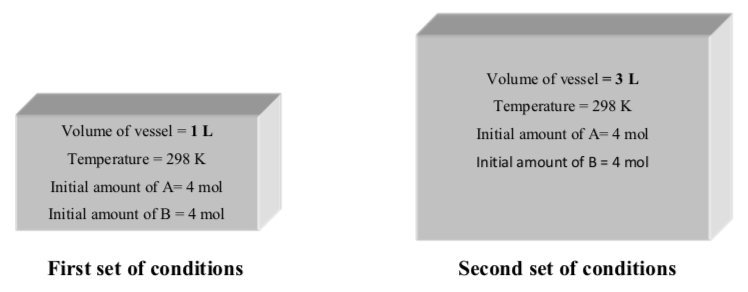
- Draw a cartoon to portray the spatial arrangement of atoms in each set of conditions:
Bunsen, a student in class, says: “The reaction under first set of conditions is fasterthan the reaction under second set of conditions”
Her friend Beaker disagrees: “No, the rates of reactions are the same because the same amount of reactants was used in both reactions”
-
What is your opinion?
-
Make an argument to convince others that your answer is correct. Include comments about collision theory. Give as much detail as you can!
Physical State and Rate of Reaction
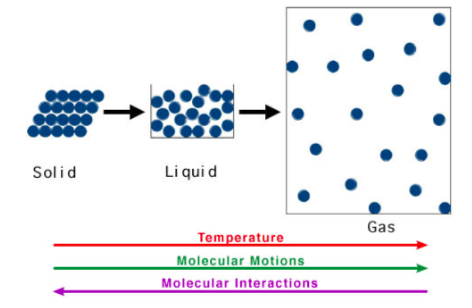
-
Rank these physical states in terms of their thermal energy (least to most): Solid Liquid Gas
Solid Liquid Gas
-
Circle the correct answers:
-
Molecules with higher thermal energy, have (more / less) molecular motion.
-
Bonds are (easier / harder) to break in molecules with higher thermal energy.
-
In a mixture of molecules at higher temperature, the proportion of reactant molecules with sufficient energy to react is (higher / lower).
-
In a gas, molecules are (closer / further apart) than in a liquid. Therefore, they are (less likely / more likely) to collide with another gaseous molecule.
-
In a solid, the molecules have (more / less) molecular motion than a liquid. Therefore, they are (less likely / more likely) to collide with another solid:

-
-
Most reactions are run as liquids or in a solvent. Explain why.
Solid phase issues
Two students are doing an experiment with equal amounts of magnesium oxide and hydrochloric acid.
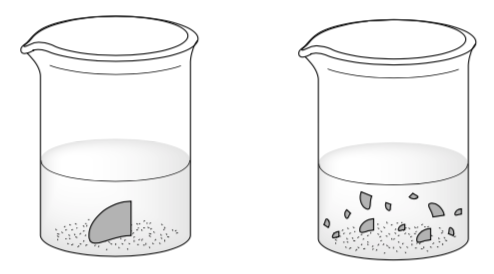
The reaction between magnesium oxide with hydrochloric acid as follows:
MgO(s) +2HCl(aq) -> MgCl2(aq)+H2O(l)
Bunsen now thinks that “powdered magnesium oxide reacts with hydrochloric acid faster than granulated magnesium oxide does”
His friend, Beaker, disagrees: “No, both reaction rates are the same, because we both used the same amounts of HCl and MgO”
-
What is your opinion?
-
What would you say to convince these students that your answer is correct? Explain using ideas of surface area and collision theory.
Reaction rate vs Reaction Time:
Reaction rate is the average change in the concentration of a reactant or a product with
time (Moles/s). Reaction time is the amount of time for the reaction to occur. Consider a reaction where two chemicals ‘A’ and ‘B’ react to form ‘C’
A(aq) +B(aq) -> C(aq)
The teacher drew a graph showing how the concentration of A changes with time.
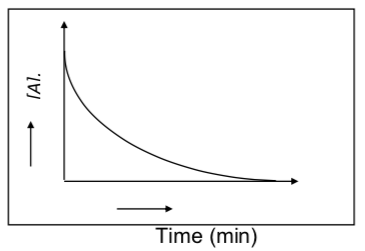
The teacher asks Bunsen and Beaker to draw a graph for the reaction rate against time.
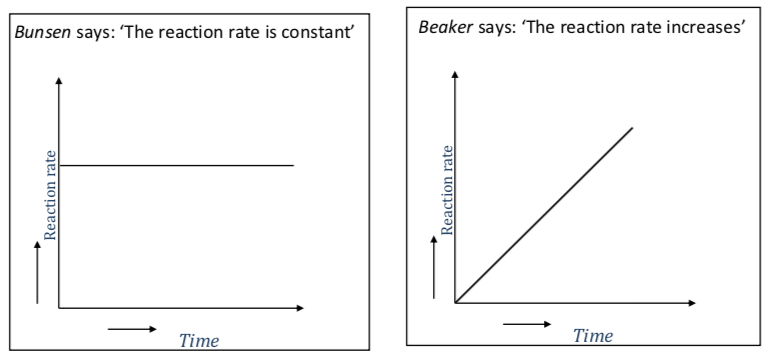
-
What is your opinion? Make a drawing to show the rate of reaction against time.
-
Explain why your answer is correct. Consider how [reactant] affects the rate. Give as much detail as you can!
Summary of Kinetics and Collision Theory
-
While __________________ determines the extent to which reactions occur,________________ is concerned with the rate of a chemical reaction.
-
The __________________ is defined as how fast or slow a reaction takes place.
-
Define Collision Theory.
-
How do the following factors affect reaction rate?
-
Temperature:
-
Presssure:
-
Concentration:o Ea:
-
Physical state:
-
-
Draw a plot that shows the concentration of a reactant vs time.
-
Draw a plot of reaction rate (Moles of reactant used/L/s) vs time.
-
Explain the following statement: Reaction rates are fastest at the beginning and slow down as the reagents are consumed.


