23.1: Relative Basicity of Amines and Other Compounds
- Page ID
- 30943
Basicity of nitrogen groups
In this section we consider the relative basicity of several nitrogen-containing functional groups: amines, amides, anilines, imines, and nitriles.
When evaluating the basicity of a nitrogen-containing organic functional group, the central question we need to ask ourselves is: how reactive (and thus how basic) is the lone pair on the nitrogen? In other words, how much does that lone pair want to break away from the nitrogen nucleus and form a new bond with a hydrogen?
Comparing the basicity of alkyl amines to ammonia
Because alkyl groups donate electrons to the more electronegative nitrogen. The inductive effect makes the electron density on the alkylamine's nitrogen greater than the nitrogen of ammonium. Correspondingly, primary, secondary, and tertiary alkyl amines are more basic than ammonia.
Comparing the basicity of alkylamines to amides
With an alkyl amine the lone pair electron is localized on the nitrogen. However, the lone pair electron on an amide are delocalized between the nitrogen and the oxygen through resonance. This makes amides much less basic compared to alkylamines.
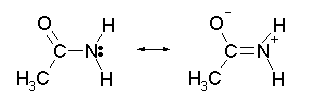
In fact,when and amide is reacted with an acid, the protonation occurs at the carbonyl oxygen and not the nitrogen. This is becase the cation resulting from oxygen protonation is resonance stabilised. The cation resulting for the protonation of nitrogen is not resonance stabilized.
Basicity of aniline
Aniline is substantially less basic than methylamine, as is evident by looking at the pKa values for their respective ammonium conjugate acids (remember that the lower the pKa of the conjugate acid, the weaker the base).
This difference is basicity can be explained by the observation that, in aniline, the basic lone pair on the nitrogen is to some extent tied up in – and stabilized by – the aromatic p system.
This effect is accentuated by the addition of an electron-withdrawing group such as a carbonyl, and reversed to some extent by the addition of an electron-donating group such as methoxide.
In the case of 4-methoxy aniline (the molecule on the left side of the figure above), the lone pair on the methoxy group donates electron density to the aromatic system, and a resonance contributor can be drawn in which a negative charge is placed on the carbon adjacent to the nitrogen, which makes the lone pair of the nitrogen more reactive. In effect, the methoxy group is 'pushing' electron density towards the nitrogen. Conversely, the aldehyde group on the right-side molecule is 'pulling' electron density away from the nitrogen, decreasing its basicity.
At this point, you should draw resonance structures to convince yourself that these resonance effects are possible when the substituent in question (methoxy or carbonyl) is located at the ortho or para position, but not at the meta position.an imine functional group is characterized by an sp2-hybridized nitrogen double-bonded to a carbon. Imines are somewhat basic, with pKa values for the protonated forms ranging around 7. Notice that this is significantly less basic than amine groups (eg. pKa = 10.6 for methylammonium), in which the nitrogen is sp3-hybridized. This phenomenon can be explained using orbital theory and the inductive effect: the sp2 orbitals of an imine nitrogen are one part s and two parts p, meaning that they have about 67% s character. The sp3 orbitals of an amine nitrogen, conversely, are only 25% s character (one part s, three parts p). Because the s atomic orbital holds electrons in a spherical shape, closer to the nucleus than a p orbital, sp2hybridization implies greater electronegative than sp3 hybridization. Finally, recall the inductive effect from section 7.3C: more electronegative atoms absorb electron density more easily, and thus are more acidic. Moral of the story: protonated imine nitrogens are more acidic than protonated amines, thus imines are less basic than amines.
Basicity of heterocyclic amines
When a nitrogen atom is incorporated directly into an aromatic ring, its basicity depends on the bonding context. In a pyridine ring, for example, the nitrogen lone pair occupies an sp2-hybrid orbital, and is not part of the aromatic sextet - it is essentially an imine nitrogen. Its electron pair is available for forming a bond to a proton, and thus the pyridine nitrogen atom is somewhat basic.
In a pyrrole ring, in contrast, the nitrogen lone pair is part of the aromatic sextet. This means that these electrons are very stable right where they are (in the aromatic system), and are much less available for bonding to a proton (and if they do pick up a proton, the aromic system is destroyed). For these reasons, pyrrole nitrogens are not strongly basic.
The aniline, pyridine, and pyrrole examples are good models for predicting the reactivity of nitrogen atoms in more complex ring systems (a huge diversity of which are found in nature). The tryptophan side chain, for example, contains a non-basic 'pyrrole-like' nitrogen, while adenine (a DNA/RNA base) contains all three types.
The lone pair electrons on the nitrogen of a nitrile are contained in a sp hybrid orbital. The 50% s character of an sp hybrid orbital means that the electrons are close to the nucleus and therefore not significantly basic.
A review of basic acid-base concepts should be helpful to the following discussion. Like ammonia, most amines are Brønsted and Lewis bases, but their base strength can be changed enormously by substituents. It is common to compare basicity's quantitatively by using the pKa's of their conjugate acids rather than their pKb's. Since pKa + pKb = 14, the higher the pKa the stronger the base, in contrast to the usual inverse relationship of pKa with acidity. Most simple alkyl amines have pKa's in the range 9.5 to 11.0, and their water solutions are basic (have a pH of 11 to 12, depending on concentration). The first four compounds in the following table, including ammonia, fall into that category.
The last five compounds (colored cells) are significantly weaker bases as a consequence of three factors. The first of these is the hybridization of the nitrogen. In pyridine the nitrogen is sp2 hybridized, and in nitriles (last entry) an sp hybrid nitrogen is part of the triple bond. In each of these compounds (shaded red) the non-bonding electron pair is localized on the nitrogen atom, but increasing s-character brings it closer to the nitrogen nucleus, reducing its tendency to bond to a proton.
| Compound | 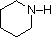 | 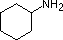 | 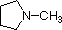 | NH3 |  | 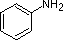 |  | 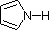 | 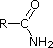 | CH3C≡N |
|---|---|---|---|---|---|---|---|---|---|---|
| pKa | 11.0 | 10.7 | 10.7 | 9.3 | 5.2 | 4.6 | 1.0 | 0.0 | -1.0 | -10.0 |
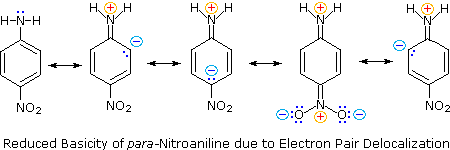
Secondly, aniline and p-nitroaniline (first two green shaded structures) are weaker bases due to delocalization of the nitrogen non-bonding electron pair into the aromatic ring (and the nitro substituent). This is the same delocalization that results in activation of a benzene ring toward electrophilic substitution. The following resonance equations, which are similar to those used to explain the enhanced acidity of ortho and para-nitrophenols illustrate electron pair delocalization in p-nitroaniline. Indeed, aniline is a weaker base than cyclohexyl amine by roughly a million fold, the same factor by which phenol is a stronger acid than cyclohexanol. This electron pair delocalization is accompanied by a degree of rehybridization of the amino nitrogen atom, but the electron pair delocalization is probably the major factor in the reduced basicity of these compounds. A similar electron pair delocalization is responsible for the very low basicity (and nucleophilic reactivity) of amide nitrogen atoms (last green shaded structure). This feature was instrumental in moderating the influence of amine substituents on aromatic ring substitution, and will be discussed further in the section devoted to carboxylic acid derivatives.
Conjugated amine groups influence the basicity of an existing amine. Although 4-dimethylaminopyridine (DMAP) might appear to be a base similar in strength to pyridine or N,N-dimethylaniline, it is actually more than ten thousand times stronger, thanks to charge delocalization in its conjugate acid. The structure in the gray box shows the locations over which positive charge (colored red) is delocalized in the conjugate acid. This compound is often used as a catalyst for acyl transfer reactions.
Finally, the very low basicity of pyrrole (shaded blue) reflects the exceptional delocalization of the nitrogen electron pair associated with its incorporation in an aromatic ring. Indole (pKa = -2) and imidazole (pKa = 7.0), see above, also have similar heterocyclic aromatic rings. Imidazole is over a million times more basic than pyrrole because the sp2 nitrogen that is part of one double bond is structurally similar to pyridine, and has a comparable basicity.
Contributors
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)