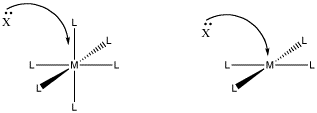LS6. Reasons for Diff. Mechanisms
- Page ID
- 4295
LS6. Some Reasons for Differing Mechanisms
We usually look for physical reasons why a given compound might undergo a reaction via one mechanism and not another. That ability adds to our understanding of chemistry. If we can take information and give it predictive value, then we may be able to make educated decisions about what is probably happening with new reactions.
Why might a reaction undergo a dissociative reaction rather than an associative one? What factors might prevent an associative pathway?
One reason may be that there is not enough room. In an associative step, an additional ligand comes in and binds to the metal. If it is already crowded, that may be difficult.
Figure LS6.1. The role of steric crowding in ligand substitution. In one of these cases, the associative mechanism is less favoured because of crowding that will occur in the transition state.
- Steric crowding may lead to a dissociative, rather than associative, mechanism.
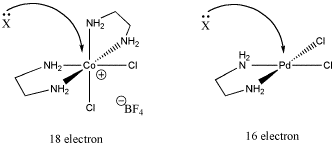
Another reason has to do with electronics. Maybe the compound cannot easily accept an additional bonding pair. That may be the case if the compound already has eighteen electrons.
Figure LS6.2. The role of electron count in ligand substitution. In one of these cases, the associative mechanism is less favoured because the metal is already electronically saturated.
- Electronic saturation may lead to a dissociative, rather than associative, mechanism.
However, if there is less crowding, and more electrons can be accommodated, an associative pathway may result.
Problem LS6.1.
i) Draw structures for the following reactions. Pay attention to geometry.
ii) Predict whether each of the substitutions would occur through associative or dissociative mechanisms.
a) AuCl3py + Na N3 → Na+ [AuCl3N3]- + py
b) Rh(C2H4)2(acac) + C2D4 → Rh(C2D4)2(acac) + C2H4
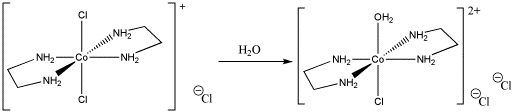
c) [Co(NH3)5Cl]2+ + H2O → [Co(NH3)5(OH2)]2+ + Cl-
d) trans-(Et3P)2PtCl2 + -SCN → trans-(Et3P)2PtCl(SCN) + Cl-
e) Rh(NH3)4Cl2+ + -CN → Rh(NH3)4Cl(CN)+ + Cl-
f) trans-[Cr(en)2Br2]+ + -SCN → cis- and trans-[Cr(en)2Br(SCN)]+ + Br-
Problem LS6.2.
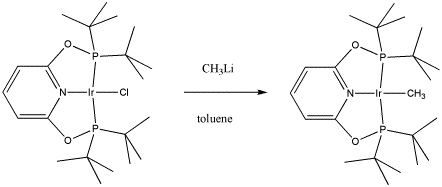
Maurice Brookhart of UNC, Chapel Hill, makes organometallic compounds in order to study fundamental questions about reactivity. In this case, he has reported making a new compound capable of "C-H activation", a reaction in which unreactive C-H bonds can be forced to break. This process holds the future promise of converting coal and natural gas into important commodities currently obtained from petroleum.
- Draw, with curved arrows, a mechanism for the ligand substitution in the synthesis of this C-H activating complex.
- Explain your reasons for your choice of reaction mechanism.
Problem LS6.3.
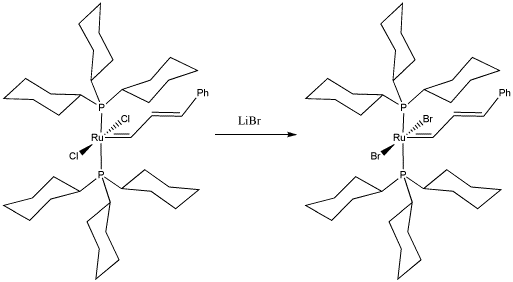
Bob Grubbs of Cal Tech was awarded the Nobel Prize in chemistry for his development of catalysts for olefin metathesis. Olefin metathesis is important both in the reforming of petroleum and in the synthesis of important commodities such as pharmaceuticals. In the following study, he replaced chlorides on a "Grubbs Generation I catalyst" to study the effect on the olefin metathesis reaction.
- Draw, with curved arrows, a mechanism for the ligand substitution in this complex.
- Explain your reasons for your choice of reaction mechanism.
- What factor(s) do you think Grubbs hoped to study by making this substitution in the catalyst?
Problem LS6.4.
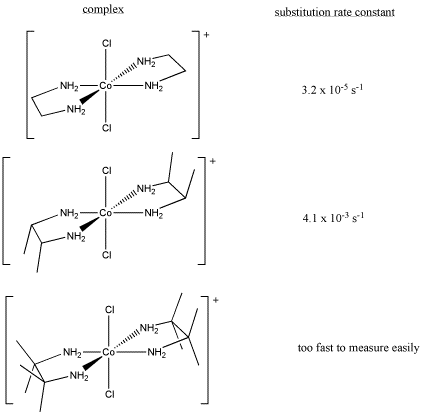
Sometimes, kinetic studies can give insight into a reaction if controlled changes in the reaction produce measurable results.
- Draw, with curved arrows, a mechanism for the ligand substitution in this reaction.
- Explain your reasons for your choice of reaction mechanism.
- Explain the following kinetic data.
Problem LS6.5.
Several different structures were proposed for Ni(cysteine)22-. Kinetic studies of substitution in this complex showed the rate was dependent in the concentration of both the metal complex and the incoming ligand. Which structure do you think is correct? Why?