Preparation of Ylides
- Page ID
- 37228
It has been noted that dipolar phosphorus and sulfur oxides are stabilized by p-d bonding. This may be illustrated by a resonance description, as shown here.
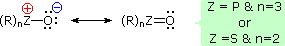
This bonding stabilization extends to carbanions alpha to phosphonium and sulfonium centers, and the zwitterionic conjugate bases derived from such cations are known as ylides. Approximate pKa's for some ylide precursors and related compounds are provided in the following table. The acidic hydrogen atoms are colored red. By convention, pKa's are usually adjusted to conform to the standard solvent water; however, in practice, measurements of very weak acids are necessarily made in non-aqueous solvents such as DMSO (dimethyl sufoxide). The green numbers in the table represent DMSO measurements, and although these are larger than the aqueous approximations, the relative order is unchanged. Note that DMSO itself is the weakest acid of those shown.
| Compound | (CH3)3S(+) I(–) | (CH3)3S(+)=O I(–) | (CH3)2S=O | (CH3)2S(=O)2 | CH3-P(OCH3)2=O |
|---|---|---|---|---|---|
| Name | trimethylsulfonium iodide |
trimethylsulfoxonium iodide |
dimethyl sulfoxide | dimethyl sulfone | dimethyl methylphosphonate |
| pKa DMSO |
20 25 |
15 18 |
30 35 |
26 31 |
25 31 |
Some characteristic preparations of ylide reagents are shown below. Very strong bases, such as butyl lithium, are required for complete formation of ylides. Sodium hydride (NaH), another powerful base, is insoluble in most solvents, but its reaction with DMSO (the weakest acid in the table) generates a strong conjugate base, CH3)S(=O)CH2(–) Na(+), known as dimsyl sodium. This soluble base is widely used for the generation of ylides in DMSO solution.
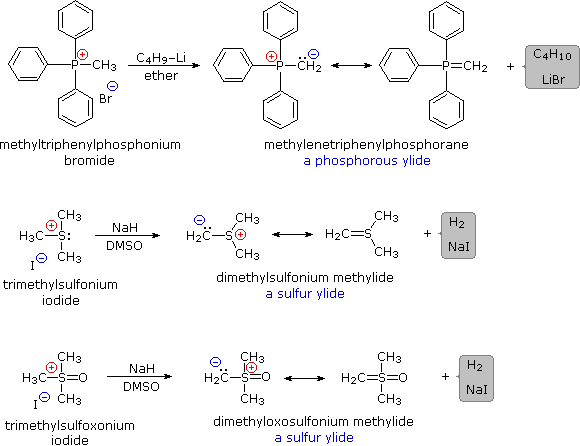
The ylides shown here are all strong bases. Like other strongly basic organic reagents, they are protonated by water and alcohols, and are sensitive to oxygen. Water decomposes alkylidenephosphoranes to hydrocarbons and phosphine oxides, as shown. Oxygen cleaves these ylides in a similar fashion, the alkylidene moiety being converted to a carbonyl compound.
R3P=CR'2 + H2O  [ R3P(OH)–CHR'2 ] [ R3P(OH)–CHR'2 ]  R3P=O + R'2CH2 R3P=O + R'2CH2 |
R3P=CR'2 + O2  R3P=O + R'2C=O R3P=O + R'2C=O |
Contributors
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry

