Making Carboxylic Acids by Oxidation of Primary Alcohols or Aldehydes
- Page ID
- 3924
Primary alcohols and aldehydes are normally oxidized to carboxylic acids using potassium dichromate(VI) solution in the presence of dilute sulfuric acid. During the reaction, the potassium dichromate(VI) solution turns from orange to green. The potassium dichromate(VI) can just as well be replaced with sodium dichromate(VI). Because what matters is the dichromate(VI) ion, all the equations and color changes would be identical.
Primary alcohols are oxidized to carboxylic acids in two stages - first to an aldehyde and then to the acid. We often use simplified versions of these equations using "[O]" to represent oxygen from the oxidizing agent. The formation of the aldehyde is shown by the simplified equation:


"R" is a hydrogen atom or a hydrocarbon group such as an alkyl group. The aldehyde is then oxidised further to give the carboxylic acid:

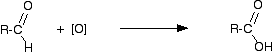
If you start with an aldehyde, you are obviously just doing this second stage. Starting from the primary alcohol, you could combine these into one single equation to give:
\[RCH_2OH + 2[O] \rightarrow RCOOH + H_2O\]
For example, if you were converting ethanol into ethanoic acid, the simplified equation would be:
\[CH_3CH_2OH + 2[O] \rightarrow CH_3COOH + H_2O\]
It is possible that you might want to write proper equations for these reactions rather than these simplified ones. You can work these out from electron-half-equations. How you do this is described in detail elsewhere on the site.
The complete equation for the conversion of a primary alcohol to a carboxylic acid is:
\[3RCH_2OH + 2Cr_2O_7^{2-} + 16H^+ \rightarrow 3RCOOH + 4Cr^{3+} + 11H_2O\]
or if you were starting from an aldehyde is:
\[3RCHO + Cr_2O_7^{2-} + 8H^+ \rightarrow 3RCOOH + 3Cr^{3+} + 4H_2O\]
It would actually be quite uncommon to make an acid starting from an aldehyde, but very common to start from a primary alcohol. The conversion of ethanol into ethanoic acid would be a typical example. The alcohol is heated under reflux with an excess of a mixture of potassium dichromate(VI) solution and dilute sulfuric acid. Heating under reflux (heating in a flask with a condenser placed vertically in it) prevents any aldehyde formed escaping before it has time to be oxidized to the carboxylic acid.
Using an excess of oxidizing agent is to be sure that there is enough oxidizing agent present for the oxidation to go all the way to the carboxylic acid. When oxidation is complete, the mixture can be distilled. You end up with an aqueous solution of the acid.
Contributors
Jim Clark (Chemguide.co.uk)


