Structure and Bonding in Ethene: The \(\pi\) Bond
- Page ID
- 875
Ethene is the formal IUPAC name for H2C=CH2, but it also goes by a common name: Ethylene. The name Ethylene is used because it is like an ethyl group (\(CH_2CH_3\)) but there is a double bond between the two carbon atoms in it. Ethene has the formula \(C_2H_4\) and is the simplest alkene because it has the fewest carbons (two) necessary for a carbon-carbon double bond.
Introduction
Bonding in carbon is covalent, containing either sigma or \(\pi\) bonds. Carbon can make single, double, or triple bonds. The number of bonds it makes determines the structure. With four single bonds, carbon has a tetrahedral structure, while with one double bond it's structure is trigonal planar, and with a triple bond it has a linear structure.
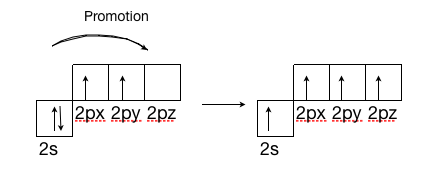
A solitary carbon atom has four electrons, two in the 2s orbital, and one in each of the 2\(p_x\) and 2\(p_y\) orbitals, leaving the \(2p_z\) orbital empty. A single carbon atom can make up to four bonds, but by looking at its electron configuration this would not be possible because there are only two electrons available to bond with. The other two are in a lone pair state, making them much less reactive to another electron that is by itself. Well it is, in order to make the four bonds, the carbon atom promotes one of the 2s electrons into the empty \(2p_z\) orbital, leaving the carbon with four unpaired electrons allowing it to now form four bonds. The electron is not promoted spontaneously. It becomes promoted when a photon of light with the correct wavelength hits the carbon atom. When this photon hits the carbon atom it gives the atom enough energy to promote one of the lone pair electrons to the \(2p_z\) orbital.
Sigma and Pi Bonds
All the bonds in Ethene are covalent, meaning that they are all formed by two adjacent atoms sharing their valence electrons. As opposed to ionic bonds which hold atoms together through the attraction of two ions of opposite charges.
Sigma bonds are created when there is overlap of similar orbitals, orbitals that are aligned along the inter-nuclear axis. Common sigma bonds are \(s+s\), \(p_z+p_z\) and \(s+p_z\), \(z\) is the axis of the bond on the xyz-plane of the atom.
\(\pi\) bonds are created when there is adequate overlap of similar, adjacent \(p\) orbitals, such as \(p_x\)+\(p_x\) and \(p_y\)+\(p_y\). Each p orbital has two lobes, one usually indicated by a + and the other indicated by a - (sometimes one may be shaded while the other is not). This + and - (shaded, not shaded) are only meant to indicate the opposite phase \(\phi\) the wave functions, they do not indicate any type of electrical charge. For a \(\pi\) bond to form both lobes of the \(p\) orbital must overlap, + with + and - with -. When a + lobe overlaps with a - lobe this creates an anti-bonding orbital interaction which is much higher in energy, and therefore not a desirable interaction.
Usually there can be no \(\pi\) bonds between two atoms without having at least one sigma bond present first. But there are special cases such as dicarbon (\(C_2\)) where the central bond is a \(\pi\) bond not a sigma bond, but in cases like these the two atoms want to have as much orbital overlap as possible so the bond lengths between the atoms are smaller than what is normally expected.
The \(\pi\) bond in ethene is weak compared to the sigma bond between the two carbons. This weakness makes the \(\pi\) bond and the overall molecule a site of comparatively high chemical reactivity to an array of different substances. This is due to the high electron density in the \(\pi\) bond, and because it is a weak bond with high electron density the \(\pi\) bond will easily break in order to form two separate sigma bonds. Sites such as these are referred to as functional groups or functionalities. These groups have characteristic properties and they control the reactivity of the molecule as a whole. How these functional groups and other reactants form various products are an important concept in organic chemistry.
Orbital Bonding in Ethene
Ethene is made up of four 1s1 Hydrogen atoms and two 2s2 2\(p_x\)1 2\(p_y\)2 carbon atoms. These carbon atoms already have four electrons, but they each want to get four more so that they have a full eight in the valence shell. Having eight valence electrons around carbon gives the atom itself the same electron configuration as neon, a noble gas. Carbon wants to have the same configuration as Neon because when it has eight valence electrons carbon is at its most stable, lowest energy state, it has all of the electrons that it wants, so it is no longer reactive.
Structure of Ethene
Ethene is not a very complicated molecule. It contains two carbon atoms that are double bonded to each other, with each of these atoms also bonded to two Hydrogen atoms.

This forms a total of three bonds to each carbon atom, giving them an \(sp^2\) hybridization. Since the carbon atom is forming three sigma bonds instead of the four that it can, it only needs to hybridize three of its outer orbitals, instead of four. It does this by using the \(2s\) electron and two of the \(2p\) electrons, leaving the other unchanged. This new orbital is called an \(sp^2\) hybrid because that's exactly what it is, it is made from one s orbital and two p orbitals.
.png?revision=1)
When atoms are an \(sp^2\) hybrid they have a trigonal planar structure. These structures are very similar to a 'peace' sign, there is a central atom with three atoms around it, all on one plane. Trigonal planar molecules have an ideal bond angle of 120° on each side.
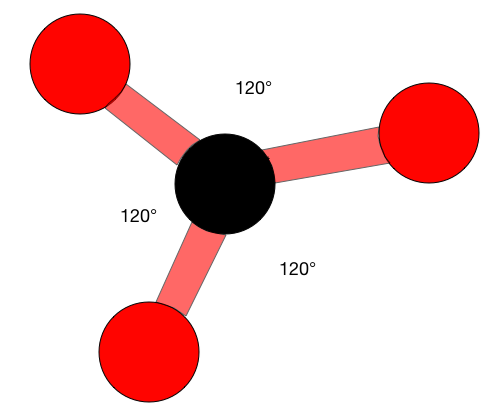
The H-C-H bond angle is 117°, which is very close to the ideal 120° of a carbon with \(sp^2\) hybridization. The other two angles (H-C=C) are both 121.5°.
Rigidity in Ethene
There is rigidity in the Ethene molecule due to the double-bonded carbons. In Ethane there are two carbons that share a single bond, this allows the two Methyl groups to rotate with respect to each other. These different conformations result in higher and lower energy forms of Ethane. In Ethene there is no free rotation about the carbon-carbon sigma bond. There is no rotation because there is also a \(\pi\) bond along with the sigma bond between the two carbons. A \(\pi\) bond is only formed when there is adequate overlap between both top and bottom p-orbitals. In order for there to be free rotation the p-orbitals would have to go through a phase where they are 90° from each other, which would break the \(\pi\) bond because there would be no overlap. Since the \(\pi\) bond is essential to the structure of Ethene it must not break, so there can be not free rotation about the carbon-carbon sigma bond.
References
- Vollhardt, K. P.C. & Shore, N. (2007). Organic Chemistry (5th Ed.). New York: W. H. Freeman.
Outside Links
- Sigma Bond: http://en.Wikipedia.org/wiki/Sigma_bond
- Pi Bond: http://en.Wikipedia.org/wiki/Pi_bond
- Ethene (Ethylene): http://en.Wikipedia.org/wiki/Ethene
- Trigonal Planar Structure & Picture: http://en.Wikipedia.org/wiki/Trigonal_planar
- http://bcs.whfreeman.com/vollhardtsc...5e/default.asp
Problems
- Write out the condensed formula for ethene.
- Write out the Kekulé formula for ethene.
- Write out the bond-line formula for ethene.
- What are the distances between carbon and hydrogen atoms when they are bonded? Carbon-carbon single bond? Carbon-carbon double bond?
- Why is it that the carbons in ethene cannot freely rotate around the carbon-carbon double bond?
Answers
1. H2CCH2
2.
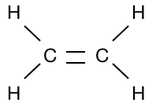
3. -
4. C-H: 1.076 angstroms, C-C: 1.54 angstroms, C=C: 1.330 angstroms
5. the carbons cannot freely rotate about the carbon-carbon double bond because in order to rotate the p-orbitals would have to pass through a 90° point where there would no longer be any overlap, so the \(\pi\) bond would have to break for there to be free rotation.

