Cadmium Sulfide
- Page ID
- 35889
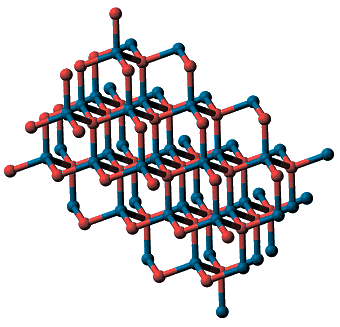
Cadmium sulfide (CdS) exists in two natural forms: greenockite and hawleyite, which differ in their crystal structure. Greenockite forms hexagonal crystals with the wurtzite structure, hawleyite has the sphalerite (zinc blende) structure.
 |
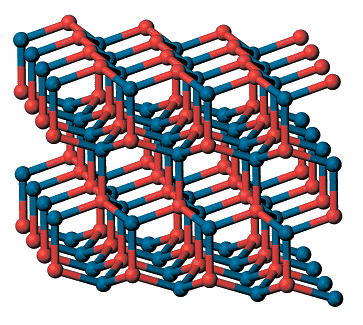 |
| Hawleyite (cubic) | Greenockite (hexagonal) |
Cadmium sulfide is a direct band gap semiconductor with Eg = 2.42 eV at room temperature. CdS is used in optoelectronics (photosensitive and photovoltaic devices). One simple use is as a photoresistor whose electrical resistance changes with incident light levels. Mixed with zinc sulfide, cadmium sulfide acts as a phosphor with long afterglow.
Cadmium sulfide was used as a pigment in paints as far back as 1819. Synthetic cadmium sulfide pigments are valued for their good thermal stability in many polymers, for example in engineering plastics. By adding selenium it is possible to obtain colors ranging from a greenish yellow to red violet.
Contributors and Attributions
- Hans Lohninger (Epina eBook Team)

