Acid-base Chemistry of Amino Acids
- Page ID
- 14650
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
Amino acids by themselves have amino (pKa ~9.0-10.5) and carboxyl groups (pKa ~2.0-2.4) that can be titrated. At neutral pH the amino group is protonated, and the carboxyl group is deprotonated. The side chains of acid and basic amino acids, and some polar amino acids can also be titrated:
| Amino acid | Functional Group | Side chain pKa |
|---|---|---|
| Cysteine | -SH | 8.3 |
| Serine | -OH | 13 |
| Threonine | -OH | 13 |
| Tyrosine | -OH | 10.1 |
| Aspartic acid | -COOH | 3.9 |
| Glutamic acid | -COOH | 4.3 |
| Histidine | Imidazole ring | 6.0 |
| Arginine | Guanidino | 12.5 |
| Lysine | -NH2 | 10.5 |
Physiological pH is near neutral. It would appear that only histidine is of physiological relevance. However, pKa values can be shifted significantly by neighboring charged groups in complex molecular structures.
Reactions of amino acids
- Free amino acids (excluding proline) share similar chemical reactivities due to the common amino and carboxyl groups.
- Different amino acid side chains have different chemical reactivities. Therefore,
- reactivities of different proteins reflects the composition of the unique sequence of amino acids in their structure.
Some common carboxyl-group reactivities:
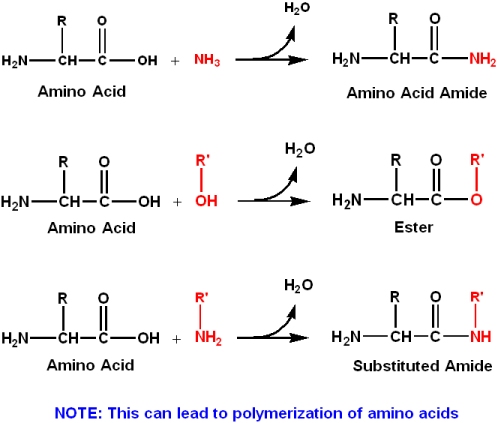
A common side chain reaction involving cysteine:
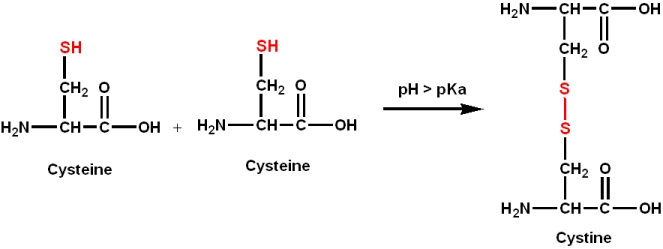
NOTE: This can covalently link two polypeptide chains in a "disulfide bond" crosslink

