Out-of-class Problems
- Page ID
- 73017
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
- What would be the effect on a gas chromatographic peak of introducing the sample at too slow a rate (i.e., making a very slow injection into a gas chromatograph from a syringe)?
- Describe the different contributions to peak broadening in a gas or liquid chromatographic system.
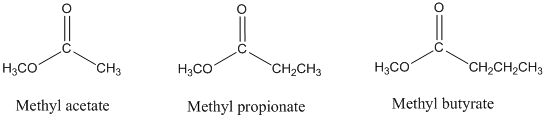
- Consider the gas chromatographic separation of the esters methyl acetate, methyl propionate, and methyl n-butyrate on a column containing a stationary phase of intermediate polarity. What would be the retention order on this column? Would this retention order change if a non-polar stationary phase had been used instead? Explain. Would the retention times change on the non-polar column? Explain.
- What would be the effect of each of the following on the plate height of a gas chromatographic column? Explain each answer by referring specifically to terms in the equation that describes chromatographic peak broadening.
- Increasing the weight of stationary phase relative to the support particles.
- Increasing the flow rate.
- Reducing the particle size of the packing.
- Decreasing the column temperature.
- Describe three general terms that can be adjusted to improve resolution in chromatographic separations and explain specific experimental changes that can be made to adjust these parameters.
- In preparing a benzene/acetone gradient for a silica gel liquid chromatographic column (note, this is silica gel and not a bonded-phase material), is it desirable to increase or decrease the proportion of benzene as the column is eluted? Explain your answer.

-
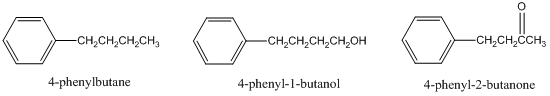
- For a reversed-phase liquid chromatographic separation on a C-18 column, predict the elution order of 4-phenylbutane, 4-phenyl-1-butanol, and 4-phenyl-2-butanone. Explain your answer.
- Suppose the liquid chromatogram of these three compounds came out as shown below. What is the problem with this chromatogram (there is a specific chromatographic term to describe it) and explain specifically what you would do experimentally to improve the chromatography?